Eli Lilly and Company facts for kids
 |
|
| Public | |
| Traded as | |
| ISIN | [https://isin.toolforge.org/?language=en&isin=US5324571083 US5324571083] |
| Industry | Pharmaceutical |
| Founded | 1876 |
| Founder | Eli Lilly |
| Headquarters | Indianapolis, Indiana, U.S. |
|
Key people
|
David A. Ricks (Chair, President, & CEO) Anat Ashkenazi (CFO) |
| Products | Pharmaceutical drugs |
| Revenue | |
|
Operating income
|
|
| Total assets | |
| Total equity | |
| Owner | Lilly Endowment (10.8%) |
|
Number of employees
|
c. 43,000 (2023) |
Eli Lilly and Company is an American company that makes medicines. Its main office is in Indianapolis, Indiana, in the U.S. The company also has offices in 18 other countries. Its medicines are sold in about 125 countries around the world.
The company was started in 1876 by Eli Lilly. He was a chemist and a veteran of the American Civil War. The company was named after him.
Eli Lilly and Company is known for making medicines that help with depression, like Prozac and Cymbalta. They also make medicines for mental health conditions, such as Zyprexa. A big part of their business comes from diabetes medicines like Humalog and Trulicity.
Lilly was the first company to make a lot of the polio vaccine that Jonas Salk developed. They also mass-produced insulin, a very important medicine for diabetes. They were one of the first companies to make human insulin using special DNA technology. This includes medicines like Humulin and Basaglar.
In 2009, Lilly agreed to pay a large amount of money to settle claims about how it marketed one of its medicines. This was a big settlement at the time.
The company is one of the largest in Indiana and gives a lot to charity there. It is also recognized as a top employer in Indianapolis for people starting their careers. Eli Lilly and Company is a member of important groups that represent medicine makers in America and Europe.
Contents
History
How the Company Started
The company's founder was Colonel Eli Lilly. He was a chemist who had served in the Union Army during the American Civil War. Colonel Lilly was the company's president until he passed away in 1898.
Before starting his own company, Eli Lilly worked in drugstores in Indiana. In 1874, he opened a drug manufacturing business with a partner. Two years later, in May 1876, he started his own company in Indianapolis. This new business became Eli Lilly and Company.
The Early Years: 1870s to 1900s
On May 10, 1876, Eli Lilly opened his own lab in Indianapolis. He started making medicines there. The sign above his shop said: "Eli Lilly, Chemist." He began with only three employees, including his son, Josiah (J. K.).
One of the first medicines Lilly made was quinine, which was used to treat malaria. By the end of 1876, the company had made $4,470 in sales. By 1879, sales grew to $48,000. In 1878, Lilly hired his brother, James, as his first full-time salesperson. Soon, the company's medicines were sold all over the country.
The company moved to bigger buildings as it grew. In 1881, it moved its main office to an industrial area in Indianapolis. Lilly also bought more places for research and making medicines.
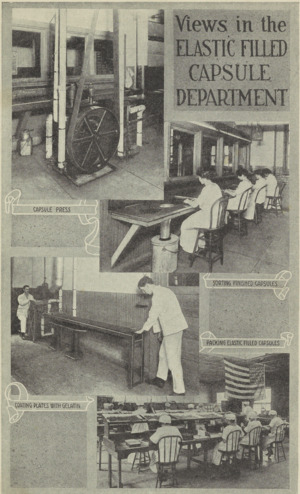
Lilly's first new idea was to put medicines inside gelatin coatings for pills and capsules. This made them easier to swallow. They also added fruit flavors and sugar coatings to pills.
In 1881, the business officially became "Eli Lilly and Company." Family members and close friends became part of the company's board. In 1882, Colonel Lilly's only son, Josiah (J. K.), joined the company after finishing college. He became the superintendent of the lab.
In 1883, the company started selling Succus Alteran. This was their first very successful product. It was sold as a "blood purifier" and helped treat some types of rheumatism and skin problems. Sales from this medicine helped Lilly expand its factories and research labs. By the late 1880s, Colonel Lilly was a top businessman in Indianapolis. His company had over 100 employees and made $200,000 in sales each year.
As Lilly grew, other businesses opened nearby. The area became a major business and industrial center. Lilly's operations in Indianapolis eventually covered more than two dozen buildings over 15 city blocks.

In 1898, Colonel Lilly's son, J. K. Lilly, became the company's president. Colonel Lilly had given him control of the business around 1890. J. K. ran the company for 34 years. Even though the economy was tough in the 1890s, the company did very well. In 1894, Lilly bought a factory just for making capsules. They also made new machines to make capsules faster. In a few years, the company was making tens of millions of capsules and pills every year.
Until the early 1900s, Lilly made and sold common medicines like "sugar-coated pills, extracts, and syrups." They used plants for their ingredients and made products by hand. Even though Lilly was careful about how they made and sold drugs, they didn't focus much on scientific research at first.
Other Lilly family members also worked for the company. Colonel Lilly's cousin, Evan Lilly, was a bookkeeper. His grandsons, Eli and Josiah Jr. (Joe), ran errands when they were boys. Both Eli and Joe joined the family business after college. They both later became company president and chairman of the board.
Josiah (J. K.), Colonel Lilly's son, became president in 1898 after his father died. At that time, the company had 2,005 different products and made over $300,000 in sales each year. Colonel Lilly was a pioneer in the modern medicine industry. Many of his early ideas became standard practice. He pushed for ethical ways of making and selling medicines. J. K. Lilly continued to support government rules for medicines. Under J. K.'s leadership, the company started using scientific ways to manage the business. They created a research department, hired more salespeople, and started selling products around the world. J. K. also oversaw a big expansion of the company. By 1905, sales reached $1 million.
Becoming Modern: 1900 to 1940
Around World War I, Eli Lilly and Company changed quickly. They expanded their factories in Indianapolis, adding new buildings for science and capsule making. In 1913, they also started building the Lilly Biological Laboratories near Greenfield, Indiana, for research and production.
The company made new medicines and improved how they made them. Lilly was a leader in making pill capsules. They were among the first to put medicines into empty gelatin capsules, which made sure each dose was exact. Lilly made capsules for themselves and sold extra ones to other companies. A 1917 article said Lilly's factory in Indianapolis was "the largest capsule factory in the world." It could make 2.5 million capsules a day. Lilly also made medicines taste better with fruit flavors and sugar coatings.
Other improvements made the factories work better and reduced mistakes. Eli Lilly, the founder's grandson, created a way to make multiple copies of drug formulas in 1909. This helped prevent errors in making medicines. In the 1920s, Eli introduced "straight-line production." This meant raw materials went in one end of the factory, and the finished product came out the other. This made production more efficient and cheaper. This new system also allowed the company to have a steady workforce, making medicines even when demand was lower.
In the 1920s, new products brought the company great success. In 1919, Josiah hired a biochemist named George Henry Alexander Clowes to lead biochemical research.
In 1921, three scientists at the University of Toronto were working on insulin to treat diabetes. Clowes suggested that Lilly work with them. The scientists were not sure about working with a company at first. But as they needed to make more insulin than their university lab could handle, Lilly and the scientists agreed to work together to mass-produce insulin. This partnership greatly sped up making large amounts of the medicine.
In 1923, Lilly started selling Iletin (Insulin, Lilly). This was the first insulin product sold in the U.S. for diabetes treatment. Banting and Macleod won a Nobel Prize in 1923 for their research. They shared it with their co-discoverers. Insulin became "the most important drug" in Lilly's history. It helped make Lilly "one of the major pharmaceutical manufacturers in the world." Lilly was the main seller of insulin in the U.S. for almost two years.
The success of insulin helped the company attract talented scientists. This led to more medical breakthroughs. By its 50th anniversary in 1926, sales reached $9 million. The company made over 2,800 different products.
In 1928, Lilly introduced Liver Extract 343 for a blood disorder called pernicious anemia. They worked with two scientists from Harvard University. In 1930, Lilly introduced Liver Extract No. 55 with a scientist from the University of Rochester. These scientists later won the Nobel Prize for their research.
Even during the Great Depression, Lilly's sales grew to $13 million in 1932. That same year, Eli Lilly, the founder's grandson, became the company's president. His father remained chairman of the board. Eli joined the company in 1909. He focused on making production more efficient and introduced many ways to save labor. He also helped expand the company's research and partnerships with university scientists.
In 1934, the company opened two new buildings: a copy of Lilly's first lab from 1876 and the new Lilly Research Laboratories. This new lab was "one of the most fully equipped facilities in the world." In the 1930s, the company also grew overseas. In 1934, Eli Lilly and Company Limited, their first international branch, was set up in England.
Growing Bigger: 1940 to 1970
World War II led to a big increase in production at Lilly. They made Merthiolate and penicillin. During the war, Lilly also worked with the American Red Cross to process blood plasma. By the end of the war, the company had dried over two million pints of blood. Merthiolate, first made in 1930, was an "antiseptic and germicide" that the U.S. army used a lot during the war. In the early 1940s, Lilly became one of the main companies making a lot of penicillin.
International business grew even more during World War II. Eli Lilly International Corp. was formed in 1943 to help with trade abroad. By 1948, Lilly employees worked in 35 countries, mostly as sales representatives in Latin America, Asia, and Africa.
After World War II, the company continued to expand. In 1945, Lilly started a big expansion project. This included two new factories in Indianapolis. They bought a large plant that used to make airplane parts. After it was fixed up in 1947, this new location made antibiotics and capsules. By 1948, Lilly had almost 7,000 employees.
Eli Lilly, who had been president since 1932, retired from managing the company in 1948. He became chairman of the board. His brother, Josiah K. Lilly Jr. (Joe), became president. During Eli's 16 years as president, sales grew from $13 million in 1932 to $117 million in 1948. Joe joined the company in 1914 and focused on employees and marketing. He was president from 1948 to 1953, then chairman until he passed away in 1966.
In 1952, the company offered its shares to the public for the first time. In 1953, Eugene N. Beesley became the new president. He was the first person outside the Lilly family to run the company.
Over the next few decades, Lilly kept developing new medicines. In the 1950s, Lilly introduced two new antibiotics: vancomycin and erythromycin. Lilly was also very involved in making and distributing Jonas Salk's polio vaccine.
In 1954, a group called the National Foundation for Infantile Paralysis asked five companies, including Lilly, to make Salk's polio vaccine for testing. Lilly was chosen partly because of its past experience working with university researchers. Lilly made 60 percent of the Salk vaccine in 1955.
Lilly continued to expand its factories outside Indianapolis. In 1950, Lilly started Tippecanoe Laboratories in Lafayette, Indiana. They increased antibiotic production there. In 1954, Lilly created Elanco Products Company to make medicines for animals.
In 1969, the company opened a new plant in Clinton, Indiana.
After a company reorganization in 1953, Lilly continued to grow worldwide. In the 1960s, Lilly had 13 related companies outside the United States. In 1962, they bought a factory in Liverpool, England. In 1968, Lilly built its first research center outside the U.S., near London, England.
From 1970 to Today
In the 1970s and 1980s, Eli Lilly and Company made many new drugs. These included an antibiotic called Keflex (1971), a heart medicine called Dobutrex (1977), and Ceclor (1979), which became the world's best-selling oral antibiotic. They also made medicines for leukemia and arthritis. When patents for older drugs ran out, many cheaper generic versions appeared. So, Lilly started to make other products like farm chemicals, animal health products, cosmetics, and medical tools.
In 1971, the company became part of the S&P 500 Index, which tracks the performance of 500 large U.S. companies.
To make its product line more diverse, Lilly bought the cosmetic company Elizabeth Arden, Inc. in 1971. Even though it lost money for five years, changes in management helped it become very successful. By 1982, its sales were up 90 percent. Sixteen years later, Lilly sold Elizabeth Arden in 1987.
In 1977, Lilly started making medical tools by buying IVAC Corporation, which makes systems for monitoring vital signs. Lilly also bought Cardiac Pacemakers Incorporated, which makes heart pacemakers, in 1977. In 1980, Lilly bought Physio-Control Corporation. Other purchases included companies that made tools for heart and blood vessels. In the early 1990s, Lilly combined these medical equipment companies into one division. This division made about 20 percent of Lilly's yearly income.
In 1989, a joint company called DowElanco was created with Dow Chemical to make farm chemicals. In 1997, Lilly sold its share of this company to Dow Chemical.
In 1994, Lilly bought PCS Systems, a company that managed prescription drug benefits. This was the largest such company in the U.S. at the time.
In 1991, Vaughn Bryson became the CEO of Eli Lilly and Company. During his time, the company reported its first quarterly loss as a public company. In 1993, Randall L. Tobias, who was on Lilly's board, became chairman, president, and CEO. He was the first leader hired from outside the company. Under Tobias, the company "cut costs and focused its mission." Lilly sold its medical equipment companies, increased international sales, bought new companies, and put more money into research. Sidney Taurel became CEO in 1998, and chairman in 1999. In 2000, Lilly reported $10.86 billion in sales.
In 1998, Lilly partnered with Icos Corporation, a biotechnology company, to develop and sell Cialis. In 2006, Lilly bought Icos to have full control of Cialis.
In January 2009, Lilly had to pay a very large fine of $1.415 billion. This was for how it marketed its medicine, Zyprexa.
In 2011, Boehringer Ingelheim and Eli Lilly and Company announced they would work together to develop and sell new medicines for diabetes.
In April 2014, Lilly announced plans to buy Swiss drugmaker Novartis AG's animal health business for $5.4 billion. This was to make its Elanco animal health unit stronger.
In March 2015, the company announced it would work with Hanmi Pharmaceutical to develop a new medicine. A day later, they announced another deal with China's Innovent Biologics to develop and sell at least three treatments. The next week, the company said it would restart its work with Pfizer on a medicine called Tanezumab.
In January 2017, Elanco Animal Health, a part of Lilly, bought Boehringer Ingelheim Vetmedica, Inc.'s pet vaccine business in the U.S. In March 2017, the company bought CoLucid Pharmaceuticals for $960 million to get a migraine medicine called lasmiditan.
In May 2018, the company bought Armo Biosciences for $1.6 billion. Days later, they announced they would buy AurKa Pharma for up to $575 million.
In January 2019, Lilly announced it would buy Loxo Oncology for about $8 billion. This greatly expanded Lilly's cancer medicine offerings. In August 2019, Elanco bought the Bayer animal health business for $7.6 billion.
In January 2020, the company announced it would buy Dermira for $1.1 billion. This gave them control of two important medicines, lebrikizumab and glycopyrronium cloth. In June 2020, the company announced it had started the world's first study of a possible monoclonal antibody treatment for COVID-19. They worked with a company called AbCellera. By August 2020, Lilly created special mobile research units (MRUs) in large trucks. These units could go to people's locations to help with clinical trials for COVID-19. In September 2020, Amgen announced they would partner with Eli Lilly to make their COVID-19 antibody treatments. In October 2020, Lilly announced that its antibody treatment was effective and asked the Food and Drug Administration for special permission to use it. In October 2020, Lilly announced it would buy Disarm Therapeutics for $135 million. In December 2020, Lilly announced it would buy Prevail Therapeutics Inc for $1 billion. This helped their work on gene therapies for brain diseases.
On April 16, 2021, the FDA removed the special permission for Lilly's antibody treatment, bamlanivimab, when used alone for mild-to-moderate COVID-19. In July 2021, the company announced it would buy Protomer Technologies for over $1 billion.
In 2022, Lilly's COVID-19 antibody drug was paused because it was not effective against the new omicron variant. A second COVID-19 antibody medicine (bebtelovimab) developed with AbCellera was given special permission in February 2022. In October, the business announced it would buy Akouos Inc. for $487 million.
Due to public pressure and new laws, Eli Lilly made insulin more affordable. They capped monthly insulin costs and lowered prices to help patients.
Mounjaro (Tirzepatide) is a medicine approved by the FDA in May 2022. It helps adults with type 2 diabetes control their blood sugar. In November 2023, the FDA approved tirzepatide for treating obesity under the name Zepbound.
On January 9, 2023, Eli Lilly and TRexBio announced they would work together on three medicines for immune-related diseases. In June, the company announced it would buy Emergence Therapeutics and Sigilon Therapeutics. In 2023, the company focused its research on medicines for obesity, diabetes, Alzheimer's, and autoimmune diseases. In July 2023, Eli Lilly announced it would buy Versanis for $1.93 billion. In October 2023, Eli Lilly acquired Point Biopharma for $1.4 billion.
In 2024, Eli Lilly announced a deal with Amazon to offer home delivery of some medicines for diabetes, obesity, and migraines through LillyDirect.
Working with Researchers
Eli Lilly and Company has a long history of working with scientists. In 1886, Ernest G. Eberhardt, a chemist, became the company's first full-time research scientist. Lilly also hired two botanists to help with early research. After World War I, the company greatly increased its scientific research and development.
The first big step came in 1919 when Josiah Lilly hired biochemist George Henry Alexander Clowes. Clowes had a lot of experience in medical research and connections to other scientists. This led to the company working with researchers in the U.S. and other countries. Clowes's first major collaboration was with the scientists who developed insulin at the University of Toronto. This partnership greatly changed the company's future. Lilly's success with making insulin made it a leading research-based medicine maker. This allowed them to hire more scientists and work with other universities on medical research. In 1934, the company built a new research lab in Indianapolis. Lilly also conducted studies at Indianapolis City Hospital to test medicines before selling them. Lilly still does clinical studies today to test medicines.
Publicly Funded Research
Besides its own research, Lilly also works on publicly funded research projects with other companies and universities. For example, they are part of a project called InnoMed PredTox. This project works with other medicine companies and research groups to make drugs safer. In 2008, this group, which included Lilly, received €8 million for a project to improve drug safety. Lilly also participates in the Innovative Medicines Initiative, a public-private research effort in Europe.
Working with Others
Eli Lilly and Company works with many different groups, including universities, medical societies, and public health organizations.
Universities
- Northern Ontario School of Medicine (NOSM) - Lilly has given money to this school.
- Population Health Research Institute (PHRI) at McMaster University - Lilly is a partner.
- University of Toronto - Lilly has given money to this university.
- University of Washington - Lilly has given between $10 million and $50 million to this school.
Medical Societies
- American Society of Hematology - Lilly is a sponsor.
- Arthritis Society - Lilly is a national partner.
- Endocrine Society - Lilly is a member of their Corporate Liaison Board.
Public Health
- Hospital for Sick Children (SickKids) - Lilly has given money to their foundation.
- Princess Margaret Cancer Centre (PMCC) - Lilly sponsors conferences and gives money to their foundation.
- Scarborough Health Network (SHN) - Lilly has given money to their foundation.
- Sinai Health Foundation - Lilly has given money to this foundation.
- Sunnybrook Health Sciences Centre - Lilly has given money to this center.
Research and Development
- Arthritis Australia - Lilly is a sponsor.
- Canadian Consortium on Neurodegeneration and Aging - Lilly is a partner.
- Colorectal Cancer Canada - Lilly is a sponsor.
- COVID-19 Therapeutics Accelerator - Lilly is a collaborator.
- Diabetes Canada - Lilly is a corporate partner.
- Juvenile Diabetes Research Foundation (JDRF) - Lilly is a corporate partner.
Medicines Made by Lilly
Before World War II, some of Lilly's most important medicines included insulin (sold as Iletin), Amytal, Merthiolate, ephedrine, and liver extracts. Iletin (Insulin, Lilly), introduced in 1923, was Lilly's first commercial insulin product.
During World War II, Lilly made penicillins and other antibiotics. They also produced "antimalarials," blood plasma, and vaccines for diseases like encephalitis and typhus.
Some of the company's more recent drug developments include cephalosporin (an antibiotic), erythromycin (another antibiotic), and Prozac (fluoxetine). Prozac is a medicine for treating clinical depression. Ceclor, introduced in the 1970s, was an oral cephalosporin antibiotic. Prozac, introduced in the 1980s, quickly became the company's best-selling product for depression. Lilly lost its U.S. patent for Prozac in 2001. Lilly is the world's largest maker and seller of medicines for many mental health conditions, including clinical depression, generalized anxiety disorder, insomnia, bipolar disorder, and schizophrenia.
In March 2023, Eli Lilly announced a $35 limit on the monthly price of insulin. This was done to follow a new law called the Inflation Reduction Act of 2022.
Past Medicines
Eli Lilly has focused on making medicines that are protected by patents. When patents expire, other companies can make generic versions.
Cialis
In 2003, Eli Lilly introduced Cialis (tadalafil). Cialis was developed with a biotechnology company called Icos Corporation. In 2006, Lilly bought Icos to have full control of the product.
Cymbalta
Cymbalta is another antidepressant made by Lilly. It is used to treat major depression and generalized anxiety. It is also used for conditions like fibromyalgia and chronic pain. Cymbalta has been one of the most successful medicines in the industry, like Prozac.
Gemzar
In 1996, the U.S. Food and Drug Administration approved Gemzar for treating pancreatic cancer. Gemzar is often used with other treatments for pancreatic cancer and also for lung cancer.
Prozac
Prozac was one of the first medicines to treat clinical depression by affecting a chemical in the brain called serotonin. The U.S. FDA approved Prozac in 1987. Generic versions became available after 2002.
Secobarbital
Eli Lilly used to make Secobarbital, a medicine that could help with sleep, reduce seizures, and calm people down. Lilly sold it under the brand name Seconal. It was used for epilepsy, temporary insomnia, and before surgery. With newer medicines available, Secobarbital is used less now, and Lilly stopped making it in 1999.
Thiomersal
Eli Lilly developed thiomersal (also called merthiolate or thimerosal), which is used to preserve vaccines. Thiomersal works by breaking down certain bacteria. It was first launched in 1930.
Zyprexa
Zyprexa (Olanzapine) is a medicine used for schizophrenia and bipolar disorder. It was released in 1996 and was the company's best-selling drug until its patent expired in 2010.
Leadership
After three generations of Lilly family members led the company, a change was made in 1944. The company was reorganized to prepare for future growth and to separate management from ownership. In 1953, Eugene N. Beesley became the first person not from the Lilly family to be the company's president.
Even though Lilly family members continued to be chairman of the board until 1969, Beesley's appointment started the shift to non-family management. Many different leaders have since run the company, including Richard Donald Wood, Vaughn Bryson, and Randall L. Tobias. Tobias was the first president and CEO hired from outside the company.
Sidney Taurel became CEO in 1998 and chairman in 1999. He retired as CEO in 2008. John C. Lechleiter then became Lilly's CEO and president in 2008.
Community Service
The Lilly family and Eli Lilly and Company have a long history of helping the community. Around 1890, Colonel Lilly gave control of the business to his son, Josiah. Colonel Lilly then focused on helping local organizations, like the Commercial Club of Indianapolis and the Charity Organization Society. Josiah's sons, Eli and Joe, also gave a lot of money to support cultural and educational groups.
Josiah Sr. continued his father's tradition of helping others. The company started sending aid to people affected by disasters. After the 1906 San Francisco earthquake, the company sent much-needed medicine. They also provided help after the 1936 Johnstown Flood.
In 1917, Lilly Field Hospital 32, named after Josiah, was set up in Indianapolis. It was moved to France during World War I and operated until 1919. During World War II, Lilly made over 200 products for the military. This included supplies for aviator survival kits and medicines for seasickness during the D-Day invasion. Lilly also dried over two million pints of blood plasma by the end of the war.
Lilly Endowment
In 1937, Josiah K. Lilly Sr. and his two sons, Eli and Joe, started the Lilly Endowment. This is a private charity foundation. They funded it with gifts of Lilly stock. The endowment still owns a part of the company today.
Eli Lilly and Company Foundation
The Eli Lilly and Company Foundation is a separate charity that the company started in 1968. It is funded by Lilly's company profits.
See also
 In Spanish: Eli Lilly and Company para niños
In Spanish: Eli Lilly and Company para niños