Atomic nucleus facts for kids
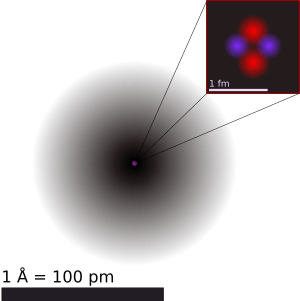
The nucleus is the tiny, dense center of an atom. Think of it like the pit of a fruit! It's made up of even smaller particles called nucleons. These nucleons are either protons or neutrons.
Around the nucleus, there's a cloud of electrons. Even though the nucleus is super small, it holds almost all of the atom's mass. Protons and neutrons are much heavier than electrons.
Neutrons have no electrical charge. Protons, however, have a positive charge. Since the nucleus is made of protons and neutrons, the whole nucleus has a positive charge. You might know that things with the same charge push each other away. This pushing force is called the electromagnetic force.
So, how does the nucleus stay together if all the positive protons are trying to push apart? There's another, much stronger force at work! It's called the strong nuclear force. This force is incredibly powerful and holds the protons and neutrons tightly together in the nucleus.
The word nucleus comes from a Latin word meaning "kernel of a nut." In 1844, Michael Faraday used it to describe the center of an atom. Later, in 1912, Ernest Rutherford suggested the modern idea of the atomic nucleus.
Contents
What is the Nucleus Made Of?
The nucleus of an atom is built from protons and neutrons. These particles are held together by the strong nuclear force. Protons and neutrons themselves are made of even smaller particles called quarks. These quarks are also held together by the strong force.
A nucleus is usually shaped like a ball, but it can sometimes be a bit stretched out or flattened. It's incredibly tiny! If an atom were the size of a football stadium, its nucleus would be like a tiny marble in the very center.
Isotopes and Nuclides
The number of neutrons in a nucleus determines its isotope. Different isotopes of the same element have the same number of protons but different numbers of neutrons. For example, all carbon atoms have 6 protons, but some carbon isotopes might have 6, 7, or 8 neutrons.
Different isotopes of an element act almost the same chemically. However, scientists can separate them. One way is using a centrifuge, like spinning clothes in a washing machine to separate water. Another way is with a mass spectrometer, which sorts atoms by their mass. These methods are used in things like making enriched uranium or in carbon dating old objects.
The total number of protons and neutrons together tells us the specific type of nucleus, called a nuclide. The total number of protons and neutrons is called the mass number. This mass number is very close to the atom's total atomic mass. Remember, electrons are so light they barely add to the atom's mass!
How We Discovered the Nucleus
Scientists didn't always know about the nucleus. The discovery of the electron by J. J. Thomson in the late 1800s was the first clue that atoms had parts inside them. At first, people thought atoms looked like a "plum pudding." This model suggested the atom was a big, positively charged ball with tiny, negatively charged electrons scattered inside, like plums in a pudding.
Around 1909, Ernest Rutherford and his team did a famous experiment. They shot tiny, positively charged particles (called alpha particles) at a very thin piece of gold foil. The "plum pudding" model predicted that these particles would mostly go straight through, maybe bending only a little.
But Rutherford was amazed by what they saw! Most particles went straight through, but a few bounced off at huge angles, and some even came straight back! It was like shooting a cannonball at tissue paper and having it bounce back.
This surprising result led Rutherford to propose a new model of the atom in 1911. He suggested that an atom has a very small, very dense center with a positive charge. This center was the nucleus! The electrons, he thought, orbited around this nucleus. This explained why some alpha particles were strongly pushed away or bounced back.
Later, in 1932, James Chadwick discovered the neutron. This discovery helped complete our modern understanding of the atom. Scientists realized that the nucleus contains both protons and neutrons, and electrons orbit outside. This model explains how atoms are built and how they behave.
Modern Nuclear Science
Today, scientists continue to study the nucleus. They use different models to understand how it works.
One idea is the liquid-drop model. It treats the nucleus like a tiny drop of liquid. This model helps explain why nuclei have a certain amount of energy and how they can split apart, a process called nuclear fission.
Another important idea is the nuclear shell model. This model is based on quantum mechanics. It helps explain why some nuclei are super stable. It's like electrons filling shells around the nucleus; protons and neutrons also fill shells inside the nucleus. When these shells are full, the nucleus is very stable. These special numbers of protons or neutrons are called magic numbers.
Scientists also study nuclei under extreme conditions. They can make nuclei spin very fast or have unusual shapes. They do this by smashing atoms together using powerful accelerators. This research helps us learn more about the strong nuclear force and how matter behaves in extreme environments.
What Happens with Nuclei?
Nuclear Decay
Sometimes, a nucleus has too many or too few neutrons. When this happens, it becomes unstable and will break down over time. This process is called nuclear decay.
For example, a nitrogen-16 atom (with 7 protons and 9 neutrons) is unstable. It will quickly change into an oxygen-16 atom (with 8 protons and 8 neutrons). During this change, one of the neutrons in the nitrogen nucleus turns into a proton and an electron. Because the number of protons changes, the atom becomes a different element! Many elements have isotopes that are stable for a very long time, even billions of years.
Nuclear Fusion
Nuclear fusion happens when two light nuclei join together to form a heavier nucleus. This process needs a huge amount of energy to push the nuclei close enough for the strong force to pull them together. That's why fusion usually happens at extremely high temperatures or pressures.
When light nuclei fuse, they release a massive amount of energy. This is because the new, heavier nucleus is more stable. Our sun and other stars are powered by nuclear fusion. They fuse hydrogen nuclei into helium, releasing the light and heat we feel. Scientists are working hard to create controlled fusion reactions on Earth to produce clean energy, like at the JET and ITER projects.
Nuclear Fission
Nuclear fission is the opposite of fusion. It's when a heavy nucleus splits into two lighter nuclei. This process also releases a lot of energy, especially for very heavy nuclei.
A special type of fission is alpha decay. In this case, a nucleus releases an alpha particle, which is like a helium nucleus.
For some very heavy nuclei, like uranium, fission can be started by hitting them with a neutron. When they split, they release more neutrons, which can then hit other nuclei and cause them to split too. This creates a chain reaction. This chain reaction is what powers nuclear power plants. It's also the source of energy for certain types of powerful bombs.
For a chain reaction to happen, there needs to be enough of the special material in one place. This is called a critical mass. Scientists have even found a place in Africa where a natural nuclear fission reactor was active over a billion years ago!
How Heavy Elements Are Made
After the Big Bang, the universe was mostly made of light elements like hydrogen (protons) and helium. Most of the heavier elements we see today were created inside stars.
Inside stars, lighter nuclei fuse together to make heavier ones. This process releases energy up to elements like nickel. To make elements heavier than nickel, stars use a different process called neutron capture. Neutrons, having no charge, can be easily absorbed by a nucleus.
There are two main ways this happens:
- The s-process (slow process): This happens in older stars over hundreds or thousands of years. It slowly builds up heavier elements like lead and bismuth.
- The r-process (rapid process): This happens in huge supernova explosions. The conditions are so extreme that nuclei quickly capture many neutrons, creating very heavy elements in just a few seconds.
Related pages
- Radioactivity
- Nuclear fusion
- Nuclear fission
- Nuclear medicine
- Nuclear physics
- Atomic number
- Atomic mass
- Isotope
- Liquid drop model
See also
 In Spanish: Núcleo atómico para niños
In Spanish: Núcleo atómico para niños
 | William L. Dawson |
 | W. E. B. Du Bois |
 | Harry Belafonte |

