International System of Units facts for kids
The International System of Units is the main way we measure things today. It's like a worldwide standard for measurements. You can call it SI for short. This comes from its French name, Système International d'unités.
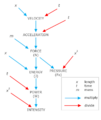
The SI system uses 7 main units. These are called base units. They are:
- The metre for length.
- The kilogram for mass.
- The second for time.
- The ampere for electric current.
- The Kelvin for temperature.
- The mole for the amount of a substance.
- The candela for brightness.
You can combine these base units to make SI derived units. These help us measure other things like volume, energy, pressure, and velocity.
Almost every country in the world uses SI. Only Myanmar, Liberia, and the United States don't use it as their official system. But even in those countries, scientists and doctors use SI all the time.
Contents
Why We Use SI
The metric system started in France after the French Revolution in 1789. At first, it only had two main units: the kilogram and the metre. Scientists really liked this new system.
In the 1860s, scientists like James Clerk Maxwell and William Thomson suggested a system with three base units: length, mass, and time. Other units would be made from these three.
Over time, scientists needed more units, especially for electricity and magnetism. By the mid-1900s, there were many different versions of the metric system. This made things confusing!
So, in 1954, a group called the General Conference on Weights and Measures (CGPM) created the first version of the International System of Units. It had six base units. The seventh base unit, the mole, was added in 1971.
Today, SI is used almost everywhere. Some countries, especially those linked to the British Empire, are slowly switching from older imperial units to the metric system. Or they use both at the same time.
Understanding SI Units
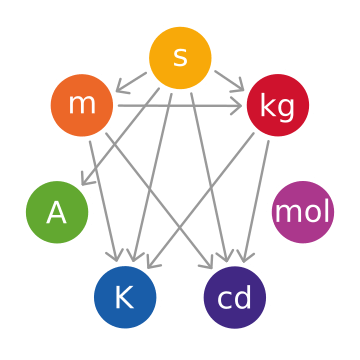
Base Units
The SI base units are the main measurements used by scientists and people worldwide. All other units, called "derived units," are made by combining these seven base units.
| Unit Name | Unit Symbol | Quantity Name | What it Measures |
|---|---|---|---|
| second | s | time | The time it takes for a special type of caesium-133 atom to vibrate 9192631770 times. |
| metre | m | length | The distance light travels in a vacuum in 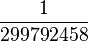 of a second. of a second. |
| kilogram | kg | mass | Defined using a very precise number called the Planck constant, along with the definitions of the metre and the second. |
| ampere | A | electric current | Based on the flow of a specific number of tiny electric charges (called the elementary charge) per second. |
| kelvin | K | temperature | Defined using the Boltzmann constant, which links temperature to energy. |
| mole | mol | amount of substance | The amount of a substance that contains exactly 6.02214076×1023 tiny particles (like atoms or molecules). This number is called the Avogadro constant. |
| candela | cd | brightness | The brightness of a light source that gives off a specific type of light at a certain power. |
Derived Units
Derived units are made by combining the base units. You can divide, multiply, or use powers of the base units to create them. Some derived units have special names to make calculations easier.
| Name | Symbol | Quantity | How it's Made (using other units) | How it's Made (using SI base units) |
|---|---|---|---|---|
| radian | rad | plane angle | − | |
| Steradian | sr | solid angle | − | |
| hertz | Hz | frequency | s−1 | |
| newton | N | force, weight | m∙kg∙s−2 | |
| pascal | Pa | pressure, stress | N/m2 | m−1∙kg∙s−2 |
| joule | J | energy, work, heat | N∙m | m2∙kg∙s−2 |
| watt | W | power, radiant flux | J/s | m2∙kg∙s−3 |
| coulomb | C | electric charge | s∙A | |
| volt | V | voltage, electrical potential difference, electromotive force | W/A J/C |
m2∙kg∙s−3∙A−1 |
| farad | F | electrical capacitance | C/V | m−2∙kg−1∙s4∙A2 |
| ohm | Ω | electrical resistance, impedance, reactance | V/A | m2∙kg∙s−3∙A−2 |
| siemens | S | electrical conductance | 1/Ω | m−2∙kg−1∙s3∙A2 |
| weber | Wb | magnetic flux | J/A | m2∙kg∙s−2∙A−1 |
| tesla | T | magnetic field strength | Wb/m2 V∙s/m2 N/A∙m |
kg∙s−2∙A−1 |
| henry | H | inductance | Wb/A V∙s/A |
m2∙kg∙s−2∙A−2 |
| degree Celsius | °C | temperature compared to 273.15 K | TK − 273.15 | K |
| lumen | lm | luminous flux | cd∙sr | cd |
| lux | lx | illuminance | lm/m2 | m−2∙cd |
| becquerel | Bq | radioactivity (how many decays per second) | s−1 | |
| gray | Gy | absorbed dose (of ionizing radiation) | J/kg | m2∙s−2 |
| sievert | Sv | equivalent dose (of ionizing radiation) | J/kg | m2∙s−2 |
| katal | kat | catalytic activity | s−1∙mol |
Prefixes
We use prefixes for very large or very small measurements. Prefixes are added to the start of a unit to make a new one. For example, kilo- means "1000 times" the original unit. So, one kilometre is 1000 metres. The prefix milli- means "0.001 times" the original unit. So, one milligram is a 1000th of a gram.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Images for kids
-
Carl Friedrich Gauss was an important mathematician and scientist.
See also
 In Spanish: Sistema Internacional de Unidades para niños
In Spanish: Sistema Internacional de Unidades para niños
 | Ernest Everett Just |
 | Mary Jackson |
 | Emmett Chappelle |
 | Marie Maynard Daly |