Nuclear physics facts for kids
Nuclear physics is a part of physics that looks closely at the center of atoms, called the atomic nucleus. It studies what these tiny centers are made of and how they interact with each other. It also explores other types of nuclear matter.
It's important not to mix up nuclear physics with atomic physics. Atomic physics studies the whole atom, including its electrons that orbit the nucleus. Nuclear physics focuses only on the nucleus itself.
The discoveries in nuclear physics have led to many useful inventions and applications. These include nuclear power plants that generate electricity and tools used in nuclear medicine, like magnetic resonance imaging (MRI) for checking inside the body. It also helps with radiocarbon dating, which scientists use to figure out the age of old things found in geology and archaeology.
Nuclear physics is closely related to particle physics, which studies even smaller particles. It also helps us understand stars and how different chemical elements were made in the universe, a field called Nuclear astrophysics.
Contents
Exploring the History of Nuclear Physics

The story of nuclear physics began in 1896. A scientist named Henri Becquerel discovered radioactivity while studying uranium salts. This was a big moment because it showed that atoms were not just solid, simple balls.
A year later, J. J. Thomson discovered the electron. This proved that atoms had smaller parts inside them. At that time, many scientists thought atoms looked like a "plum pudding." They imagined a positively charged ball with tiny, negatively charged electrons stuck inside it.
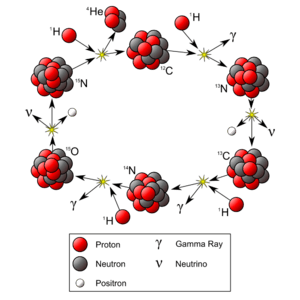
Many scientists, including Marie and Pierre Curie, and Ernest Rutherford, studied radioactivity more deeply. By the early 1900s, they found three main types of radiation coming from atoms: alpha, beta, and gamma radiation.
In 1911, Otto Hahn and later James Chadwick in 1914 found something puzzling about beta decay. The electrons released had a continuous range of energies. This seemed to break the rule of energy conservation at the time.
Becquerel, Marie Curie, and Pierre Curie won the Nobel Prize in Physics in 1903 for their work on radioactivity. Rutherford won the Nobel Prize in Chemistry in 1908 for his research on how elements break down.
In 1905, Albert Einstein came up with his famous idea of mass–energy equivalence. This idea, expressed as E=mc², explained that mass and energy are related. It helped explain where the huge energy from radioactivity came from, once scientists understood that the nucleus itself was made of smaller parts called nucleons.
Understanding Atoms
What Are Atoms Made Of?
Atoms are the tiny building blocks of everything around us. They are made of three main parts: electrons, neutrons, and protons.
The protons and neutrons are found in the very center of the atom. This central part is called the nucleus. The nucleus is the heaviest part of the atom and holds most of its mass.
The electrons are much lighter and move very quickly around the nucleus. They form what is known as an electron cloud. This cloud takes up most of the space in an atom, even though it has very little mass.
Protons have a positive charge, and electrons have a negative charge. These opposite charges attract each other, which is how the atom stays together. Neutrons have no charge.
Key Features of Atoms
Atoms have different features that help us tell them apart and understand how they behave. These features include:
- Atomic number: This is the number of protons in an atom's nucleus. It defines what element the atom is.
- Mass number: This is the total number of protons and neutrons in an atom's nucleus.
- Isotopes: These are atoms of the same element that have the same number of protons but different numbers of neutrons.
Forces Inside an Atom
There are three main forces that work inside an atom to keep it together and control its behavior:
- Electromagnetic force: This force keeps the negatively charged electrons orbiting around the positively charged nucleus.
- Strong force: This is the strongest force in the universe! It holds the protons and neutrons tightly together inside the nucleus, even though protons naturally repel each other because they are all positively charged.
- Weak force: This force controls how atoms can change or "decay" into other types of atoms.
Quantum Theory and Atoms
In the early 20th century, scientists found it hard to explain how atoms behaved using their old ideas about matter. So, they created a completely new way to understand matter and energy, called quantum theory.
Quantum theory helps us understand that tiny particles, like those in an atom, can act both like a particle (a tiny bit of matter) and a wave (like light or sound).
How Atoms Emit Radiation
Atoms can give off radiation when their electrons lose energy. When an electron loses energy, it drops from a higher energy level (or orbital) to a lower one.
The amount of energy lost by the electron determines the wavelength of the radiation. This radiation can be seen as visible light, or it can be other types of radiation with shorter wavelengths, like X-rays.
Images for kids
See also
 In Spanish: Física nuclear para niños
In Spanish: Física nuclear para niños