History of spectroscopy facts for kids
Spectroscopy is a science that studies how light and matter interact. It helps us understand what things are made of and how they behave by looking at the "fingerprints" of light they give off or absorb. This field started becoming important in the 1600s when new tools like prisms allowed scientists to look closely at the Sun's light.
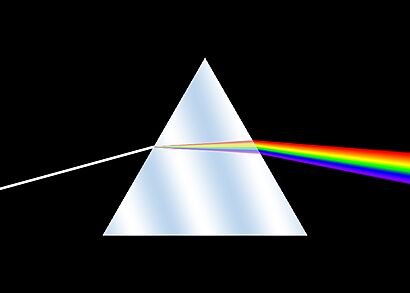
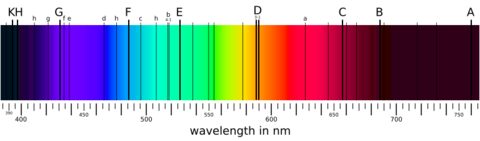
Isaac Newton was the first to use the word spectrum to describe the band of colors, like a rainbow, that make up white light. Later, in the early 1800s, Joseph von Fraunhofer made spectroscopy much more accurate. Since then, spectroscopy has been super important in chemistry, physics, and astronomy. Fraunhofer even found dark lines in the Sun's spectrum, which are named after him!
Contents
How Spectroscopy Began and Grew
People knew about prisms making rainbows even in Roman times. While Isaac Newton is often called the founder of spectroscopy, other scientists like Athanasius Kircher and Robert Boyle studied the solar spectrum before him. Newton published his famous book Opticks, explaining how light spreads out, a process called dispersion.
His experiments showed that a prism could split white light into different colors. He also proved that these colors could be put back together to make white light again. This meant the prism wasn't creating the colors, but just separating what was already there in white light. Newton's idea that light was made of tiny particles slowly changed to the wave theory of light.
It took until the 1800s for scientists to start measuring dispersed light in a standard way. Newton used light from flames and stars, including the Sun, for his experiments. Later studies showed that different chemicals create unique patterns of color when heated, like when salts were added to alcohol flames.
Early 1800s: New Discoveries
In 1802, William Hyde Wollaston improved Newton's spectrometer. He added a lens to focus the Sun's spectrum. Wollaston noticed that the colors weren't spread evenly. There were dark bands where some colors seemed to be missing. He thought these were natural borders between colors, but this idea was later proven wrong.
Joseph von Fraunhofer made a big step forward. Instead of a prism, he used a diffraction grating to spread out light. A diffraction grating has thousands of tiny, closely spaced slits. This tool made the light separation much clearer and allowed scientists to measure the exact wavelengths of light.
Fraunhofer's work helped scientists compare spectra from different labs and sources. He carefully observed and published the dark bands in the Sun's spectrum. These are still called Fraunhofer lines today.
Around the same time, scientists like John Herschel and William H. F. Talbot used flame spectroscopy to study different salts.
Mid-1800s: Linking Elements to Light
In 1835, Charles Wheatstone found that different metals could be told apart by the bright lines in the light from their sparks. This was a new way to identify materials.
In 1849, J. B. L. Foucault showed that absorption (dark) lines and emission (bright) lines at the same wavelength come from the same material. The difference depends on the temperature of the light source.
Anders Jonas Ångström, a Swedish physicist, also studied gas spectra. He suggested that a hot gas gives off light at the same wavelengths it can absorb. He also measured the emission spectrum of hydrogen, which later became known as the Balmer lines.
The biggest breakthrough in linking spectra to chemical elements came in the 1860s. German physicists Robert Bunsen and Gustav Kirchhoff found that the dark Fraunhofer lines in the Sun's spectrum matched the bright lines they saw from elements in their lab. This was huge for both lab work and astrophysics (studying stars and space).
Bunsen and Kirchhoff used Fraunhofer's optical tools and Bunsen's improved flame source. They carefully studied the spectra of many chemicals. They proved that each chemical element has its own unique spectral pattern, like a fingerprint. This created the field of analytical spectroscopy. They even discovered new chemical elements using this method!
Kirchhoff also developed three important laws of spectroscopy:
- A very hot solid, liquid, or gas under high pressure gives off a continuous spectrum (all colors).
- A hot gas under low pressure gives off a "bright-line" or emission-line spectrum (only specific colors).
- If you look at a continuous spectrum through a cool, low-density gas, you'll see an absorption-line spectrum (dark lines where the gas absorbed light).
In the 1860s, William and Margaret Huggins used spectroscopy to figure out that stars are made of the same elements as Earth. They also used the Doppler shift to measure how fast stars like Sirius were moving. They were the first to take a spectrum of a planetary nebula, like the Cat's Eye Nebula, showing that nebulae are different from stars.
August Beer noticed how light absorption relates to how much of a substance is present. This led to the creation of the spectrophotometer, a device that measures light absorption very accurately.
Late 1800s: Exploring New Wavelengths
The late 1800s brought many new tools and discoveries. Photography helped record spectra. Rowland invented the concave diffraction grating, which was even better for spreading light. Schumann explored vacuum ultraviolet (VUV) light, which is light with very short wavelengths.
Scientists like Balmer found mathematical relationships for the wavelengths of light in the hydrogen spectrum. Maxwell put together all the knowledge about electromagnetic waves into his famous equations.
In 1895, Wilhelm Conrad Röntgen discovered X-rays, which are now used in X-ray spectroscopy. A year later, Antoine Henri Becquerel discovered radioactivity, and Pieter Zeeman saw that magnetic fields could split spectral lines. In 1897, Joseph Larmor explained this splitting by saying electrons in atoms oscillate.
Early 1900s: Quantum Ideas and New Models
The early 1900s brought the start of quantum theory from Planck and Einstein. This helped explain why atoms give off light in specific patterns. Scientists like Lyman and Paschen found more spectral series for hydrogen in different light ranges.
In 1912, John William Nicholson created an atomic model that included both a nucleus and quantum ideas. He showed how electron movements in his atom matched the spectral lines seen from the Sun and nebulae.
In 1913, Bohr developed his quantum model of the atom. This model could explain the specific wavelengths of light emitted when electrons in hydrogen atoms moved between different energy levels. Later, in 1937, "E. Lehrer created the first fully-automated spectrometer," making measurements even more accurate. With better tools like photo-detectors, scientists could measure how much light substances absorbed at specific wavelengths.
Infrared and Raman Spectroscopy
Early scientists studying infrared (IR) spectra had to build their own instruments, which made getting accurate measurements hard. During World War II, the U.S. government needed to make rubber. This required analyzing different types of hydrocarbons. Companies began developing optical instruments, leading to the first commercial infrared spectrometers. These tools made Infrared Spectroscopy a popular way to find the "fingerprint" of any molecule.
Raman spectroscopy was first observed in 1928 by Sir Chandrasekhara Venkata Raman. It's based on the Raman effect, where light changes its color slightly when it interacts with a substance. The pattern of this change is unique for each compound, helping scientists identify them.
Laser Spectroscopy
Laser spectroscopy uses lasers to study the light emitted by matter. The idea for the laser came from its older cousin, the maser, which was invented by Charles H. Townes. Masers stimulated matter to find the specific radio frequencies atoms and molecules emitted. Townes realized that more accurate detections were possible with higher frequencies. This led to the idea of using visible and infrared light for spectroscopy, which became possible with the help of Arthur Leonard Schawlow.
Lasers have greatly improved experimental spectroscopy. Laser light allows for super precise experiments, like studying how light interacts with other things. They also help accurately detect specific wavelengths and frequencies, leading to inventions like laser atomic clocks. Lasers also made time-based spectroscopy more accurate by measuring the speed or decay times of photons.
Laser spectroscopy has many uses. For example, it can detect compounds in materials. One method, called Laser-induced Fluorescence Spectroscopy, can tell what a solid, liquid, or gas is made of in situ (right where it is). This means you don't have to take the material to a lab for testing!
See also
 In Spanish: Historia de la espectroscopia para niños
In Spanish: Historia de la espectroscopia para niños
- List of spectroscopists
- Mass spectrometry
- History of quantum mechanics
Images for kids