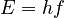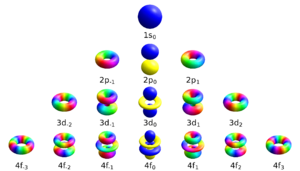History of quantum mechanics facts for kids

The history of quantum mechanics is a super important part of how we understand modern physics. It all started with scientists trying to explain strange things like how hot objects glow, how light can knock electrons out of metal, and the colors that gases give off. This early time is called the Old Quantum Theory.
Later, around 1925, scientists like Erwin Schrödinger and many others developed "modern" quantum mechanics. This new way of thinking helped us understand tiny particles and forces much better. The term "quantum mechanics" was first used by a group of physicists, including Max Born, Werner Heisenberg, and Wolfgang Pauli, in Germany in the early 1920s.
The word quantum comes from a Latin word meaning "how much." When something is "quantized," it means it can only have specific, fixed values, not just any value. Think of money: you can have 1 dollar or 2 dollars, but not 1.5 dollars (unless you count cents, which are also fixed amounts!). Mechanics is the study of how forces make objects move. So, quantum mechanics is about how things move when their properties, like energy, are quantized.
Contents
How Physics Changed: The End of the Classical Era
Many discoveries and problems in the 1800s set the stage for quantum mechanics to appear.
Light as a Wave
For a long time, scientists argued about whether light was made of tiny particles or waves. In the 1600s, Isaac Newton thought light was particles because it traveled in straight lines. But others, like Christiaan Huygens, thought it was waves.
Later, in 1801, Thomas Young did his famous double-slit experiment. This showed that light could create interference patterns, just like waves in water. This proved that light acts like a wave.
Then, James Clerk Maxwell discovered that light is actually a type of electromagnetic wave. This means light is made of vibrating electric and magnetic fields. This idea was a huge step towards understanding quantum mechanics.
The Idea of Atoms
In the early 1800s, scientists like John Dalton and Amedeo Avogadro showed that matter is made of tiny particles called atoms. Later, scientists like James Clerk Maxwell and Ludwig Boltzmann used this idea to create the kinetic theory of gases. This theory explained how gases behave by imagining them as many tiny atoms moving around.
Even though the idea of atoms was helpful, there were still some things classical physics couldn't explain. For example, how the temperature of gases affected their properties. In 1877, Ludwig Boltzmann even suggested that the energy levels of a molecule might be "discrete," meaning they could only have specific values, not just any value. This was an early hint of quantization.
Discovering Electrons
At the end of the 1800s, J. J. Thomson discovered electrons. He found that electrons are tiny particles with a negative charge, much lighter than atoms. Scientists soon realized that every atom contains many of these electrons.
Problems with Radiation Theory
Scientists in the 1800s also studied the light given off by hot objects, like flames or the Sun. They noticed that the color and brightness of this light changed with temperature.
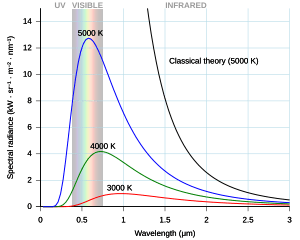
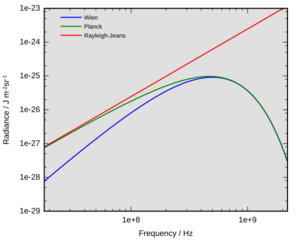
A "black body" is an ideal object that absorbs all light that falls on it and emits light only because of its temperature. The light it emits is called "black-body radiation." Classical physics could only explain parts of this radiation, but not the whole picture. For example, the Rayleigh–Jeans law worked well for long wavelengths but predicted that hot objects should give off an infinite amount of energy at short wavelengths. This was clearly wrong and was called the "ultraviolet catastrophe." Scientists needed a new theory to explain everything.
Old Quantum Theory: New Ideas Emerge
Quantum mechanics developed in two main stages. The first stage, called the old quantum theory, started around 1900. It brought totally new ways of thinking to explain things that classical physics couldn't.
Max Planck and Energy Quanta
When you heat something up, it starts to glow. First red, then yellow, white, and even blue as it gets hotter. This glow is called thermal radiation.
In 1900, Max Planck came up with a groundbreaking idea to explain black-body radiation. He suggested that energy is not given off smoothly but in tiny, fixed packets, or "quanta." Each packet of energy for a specific type of light was proportional to its frequency. The constant that links them is now called the Planck constant.
Planck's idea was the first "quantum theory" in physics. He won the Nobel Prize in 1918 for this discovery. At first, Planck thought this was just a mathematical trick, but it turned out to be a fundamental truth about our universe.
Albert Einstein and the Photoelectric Effect
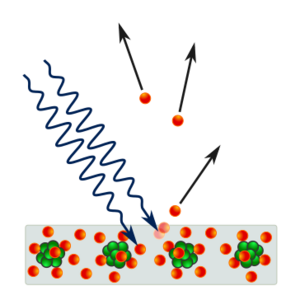
In 1887, Heinrich Hertz noticed that shining light on a metal surface could make it release electrons. This is called the photoelectric effect. Ten years later, J. J. Thomson confirmed these were electrons. In 1902, Philipp Lenard found something strange: the energy of the released electrons didn't depend on how bright the light was, but only on its color (frequency). This didn't make sense with classical physics.

In 1905, Albert Einstein used Planck's idea to explain the photoelectric effect. He suggested that light itself is made of tiny packets of energy, which he called "light quanta" (later called photons). He said that when a photon hits an electron, it gives all its energy to that electron.
The energy of a single photon is given by:
Here, E is energy, h is Planck's constant, and f is the light's frequency (its color).
Einstein explained that it takes a certain amount of energy, called the "work function" (φ), to remove an electron from a metal. If a photon's energy (hf) is less than the work function, no electron will be released. If it's more, the electron will be released, and any extra energy becomes the electron's movement energy. This explained why only the light's frequency, not its brightness, determined the electron's energy. Einstein's idea was revolutionary!
Niels Bohr and the Quantized Atom
By the early 1900s, scientists knew that atoms had a tiny, positively charged center called the nucleus, with negatively charged electrons orbiting around it, like planets around the Sun. This was called the Rutherford model. But there was a problem: classical physics said that orbiting electrons should constantly lose energy and spiral into the nucleus, making atoms unstable. This clearly wasn't happening.
Another puzzle was the emission spectrum of atoms. When gases are heated, they don't glow with all colors. Instead, they give off light only at very specific colors, creating distinct lines in a spectrum. For example, hydrogen gas glows with four specific colors in the visible spectrum.
{{wide image|Emission spectrum-H.svg |757px|The Emission spectrum of hydrogen. When heated, hydrogen gas gives off light in four distinct colors (spectral lines) that we can see, plus other colors in infrared and ultraviolet light.]]
In 1913, Niels Bohr proposed a new model for the atom. He suggested that electrons could only orbit the nucleus at certain fixed distances, like steps on a ladder. They couldn't be in between these steps. When an electron moved from a higher energy orbit to a lower one, it would instantly jump and release a photon of light with a specific energy (and thus a specific color). This explained why atoms only emit light at certain frequencies.
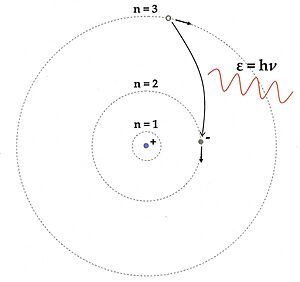
Bohr's model was a huge success because it explained the hydrogen spectrum using known constants. It also showed why electrons don't crash into the nucleus: they can't lose energy continuously. However, Bohr's model couldn't explain atoms with more than one electron or why some spectral lines were brighter than others. Even though some parts of Bohr's model were later proven wrong, the main idea that electron energies in atoms are quantized was correct.
Electron Spin
In 1922, Otto Stern and Walther Gerlach did an amazing experiment. They shot a beam of silver atoms through a special magnetic field. Classical physics predicted that the atoms would spread out evenly. But instead, they saw the atoms split into two distinct beams, one going up and one going down.
This meant that a property of the atoms, related to their magnetic behavior, could only point in two specific directions (up or down), not just any direction. This property was later called "spin."
In 1925, scientists George Uhlenbeck and Samuel Goudsmit suggested that electrons behave as if they are spinning, like tiny tops. This "spin" creates a small magnetic field and helps explain why electrons behave the way they do in atoms.
Louis de Broglie and Matter Waves

In 1924, Louis de Broglie had a brilliant idea: if light can act like both a wave and a particle, maybe matter (like electrons) can too! He suggested that every moving particle has a wave associated with it. The wavelength of this "matter wave" depends on the particle's momentum (how heavy it is and how fast it's moving).
De Broglie's idea helped explain Bohr's orbits. He suggested that an electron in an orbit around a nucleus acts like a standing wave. Think of a guitar string: it can only vibrate at certain wavelengths to create a stable sound. Similarly, an electron can only exist in orbits where its matter wave forms a stable standing wave. This means only certain orbits are allowed, explaining why electron energies are quantized.
Three years later, in 1927, scientists proved de Broglie right! Clinton Joseph Davisson and Lester Halbert Germer showed that electrons could be diffracted, just like light waves. This experiment confirmed that matter indeed has wave-like properties. De Broglie won the Nobel Prize in Physics in 1929 for his idea.
Modern Quantum Mechanics Takes Shape
De Broglie's idea of matter waves led to the birth of modern quantum mechanics around 1925.
Matrix Mechanics
In 1925, Werner Heisenberg, along with Max Born and Pascual Jordan, developed a new way to describe quantum mechanics called matrix mechanics. This method used complex mathematical tables (matrices) to calculate how particles behave and how their properties change.
Heisenberg also came up with an early version of the uncertainty principle in 1927. This principle states that you cannot know both the exact position and the exact momentum (speed and direction) of a particle at the same time. The more precisely you know one, the less precisely you can know the other.
Wave Mechanics
In 1926, Erwin Schrödinger developed another way to describe quantum mechanics, based on de Broglie's matter waves. He created the Schrödinger equation, which describes how the "wave function" of a particle changes over time. The wave function is a mathematical description of a particle's quantum state. Schrödinger said that the wave function helps predict the probability of finding a particle in a certain place or with a certain energy.
Schrödinger's equation could accurately calculate the energy levels of hydrogen atoms, just like Bohr's model. Soon after, Schrödinger showed that his wave mechanics and Heisenberg's matrix mechanics were actually two different ways of looking at the same thing; they gave the same results.
The Copenhagen Interpretation
Scientists like Bohr and Heisenberg tried to understand what these new mathematical models really meant for how the universe works. Their ideas are often called the Copenhagen interpretation. Here are some key ideas:
- Quantum State: A system is fully described by its "quantum state."
- Wave Equation: The Schrödinger equation shows how this quantum state changes over time, giving matter and light wave-like features.
- Probabilities: We can't always know exactly what will happen. Instead, quantum mechanics tells us the probability of different outcomes. For example, it tells us the chance of finding an electron in a certain spot.
- Uncertainty: You can't know certain pairs of properties (like position and momentum) at the same time with perfect accuracy. This is Heisenberg's uncertainty principle.
- Wave-Particle Duality: Both matter and light can act like waves and particles. An experiment might show one side, but not both at the same time. This is Bohr's Complementarity principle.
Understanding the Hydrogen Atom with Modern Quantum Mechanics
Bohr's model imagined electrons orbiting like planets. But the uncertainty principle tells us that electrons don't have exact paths like planets. Instead, electrons exist in "atomic orbitals." An orbital is like a fuzzy cloud that shows where an electron is most likely to be found. It's a probability distribution, not a fixed path.
Schrödinger's equation can calculate the shapes and energies of these orbitals. It accurately matches the energy levels found in the Bohr model. Each electron in an atom has four main properties, called quantum numbers:
- Principal Quantum Number (n): This tells us the electron's main energy level and how far it is from the nucleus. Higher numbers mean higher energy and farther away. (n = 1, 2, 3...)
- Azimuthal Quantum Number (l): This describes the shape of the electron's orbital. Different shapes are called s, p, d, f, etc. (l = 0, 1, ..., n-1).
- Magnetic Quantum Number (ml): This describes the orbital's orientation in space, like how it's tilted. (ml = -l, ..., 0, ..., l).
- Spin Quantum Number (ms): This describes the electron's "spin" (either +1/2 or -1/2).
The Pauli exclusion principle states that no two electrons in an atom can have the exact same set of all four quantum numbers. This rule helps explain how electrons fill up orbitals and why the periodic table is organized the way it is.
Dirac, Relativity, and New Math
Around 1927, Paul Dirac started combining quantum mechanics with special relativity, Einstein's theory about space and time. He created the Dirac equation, which described electrons in a way that fit with relativity. This equation even predicted the existence of the positron, an "anti-electron," which was later discovered! Dirac also developed new mathematical tools that are still widely used today.
Quantum Field Theory
After 1927, scientists began applying quantum mechanics not just to single particles, but to "fields" that fill space. This led to quantum field theory. A big success was quantum electrodynamics (QED), developed by Richard Feynman, Julian Schwinger, and Shin'ichirō Tomonaga in the 1940s. QED describes how electrons, positrons, and light interact. It became a model for other quantum field theories.
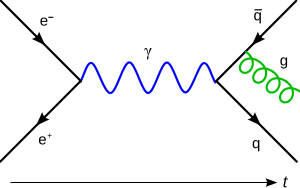
Later, scientists developed quantum chromodynamics (QCD), which describes the strong force that holds atomic nuclei together. In the 1970s, scientists like Sheldon Glashow, Steven Weinberg, and Abdus Salam showed how the weak nuclear force and electromagnetism could be combined into a single "electroweak force."
Quantum Information
In recent decades, a new field called quantum information science has emerged. This area explores how quantum mechanics can be used for things like super-fast computers (quantum computing) and ultra-secure communication (quantum key distribution).
Important Experiments in Quantum Mechanics
Here are some key experiments that helped us understand the quantum world:
- Thomas Young's double-slit experiment (around 1801): Showed that light acts like a wave.
- J. J. Thomson's cathode ray tube experiments (1897): Discovered the electron.
- Studies of black-body radiation (1850-1900): Couldn't be explained without quantum ideas.
- The photoelectric effect (explained by Einstein in 1905): Showed that light comes in energy packets (photons).
- Robert Millikan's oil-drop experiment (1909): Showed that electric charge comes in fixed units.
- Ernest Rutherford's gold foil experiment (1911): Showed that atoms have a tiny, dense nucleus.
- James Franck and Gustav Hertz's electron collision experiment (1914): Showed that atoms absorb energy in fixed amounts.
- Otto Stern and Walther Gerlach's Stern–Gerlach experiment (1920): Showed that particle spin is quantized.
- Clinton Davisson and Lester Germer's electron diffraction experiment (1927): Showed that electrons act like waves.
- Carl David Anderson's discovery of the positron (1932): Confirmed Dirac's prediction.
- Claus Jönsson's double-slit experiment with electrons (1961): Further confirmed the wave nature of electrons.
- The quantum Hall effect (1980): Led to new ways to measure electrical resistance very precisely.
- Experiments verifying quantum entanglement (1972): Showed that linked particles can affect each other instantly, no matter how far apart they are.
|
See also
 In Spanish: Historia de la mecánica cuántica para niños
In Spanish: Historia de la mecánica cuántica para niños
- Golden age of physics
- Einstein's thought experiments
- History of quantum field theory
- History of chemistry
- History of molecular theory
- History of thermodynamics
- Timeline of atomic and subatomic physics