History of thermodynamics facts for kids
The history of thermodynamics is about how we learned about heat, energy, and how things move. It's a big part of the history of physics and chemistry. Understanding thermodynamics helped us create amazing technologies like the steam engine, internal combustion engines (like in cars), and even how we make electricity. It also helped us understand tiny atoms and how they behave.
Contents
Ancient Ideas About Heat
Long ago, people thought heat was connected to fire. Around 3000 BC, the ancient Egyptians linked heat to their stories about how the world began. In ancient Indian philosophy, people believed that five main elements – earth, water, air, fire, and space – made up everything.
In ancient Western thinking, a philosopher named Empedocles suggested that all things came from four elements: earth, water, air, and fire. His idea of fire was similar to later concepts like "phlogiston" and "caloric," which were early theories about heat. Another Greek philosopher, Heraclitus, was famous for saying "All things are flowing," and he also believed fire, earth, and water were key elements.
What is a Vacuum?
In the 5th century BC, the Greek philosopher Parmenides thought that a vacuum (an empty space with nothing in it) couldn't exist. Aristotle agreed with him. However, other thinkers like Leucippus and Hero of Alexandria disagreed. For a long time, people argued about whether a vacuum was possible and tried to create one, but they didn't succeed.
The Idea of Atoms
The idea that everything is made of tiny, unseen particles called atoms is very important to thermodynamics today. Ancient thinkers like Leucippus and Democritus first suggested atomism. Later, the Epicureans also supported this idea. For a long time, the atomic theory was mostly a philosophical idea. It wasn't until the 20th century that scientists found experimental proof that atoms really exist.
Discoveries in the 1600s
Early Thermometers
In the 16th and 17th centuries, European scientists like Galileo Galilei and Santorio Santorio created simple air thermometers. These devices could tell if the air was getting hotter or colder. They might have been inspired by even older devices that could make air expand and contract.
Heat is Motion
The idea that heat is a form of motion has been around for a long time. The English philosopher Francis Bacon wrote about it in 1620. He believed that "Heat itself, its essence... is motion and nothing else." This means he thought heat was caused by tiny particles moving around.
Boyle's Law and Air Pumps
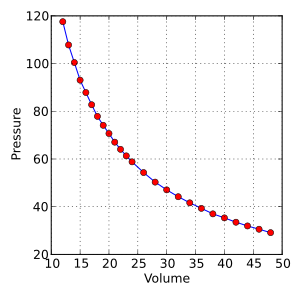
In 1656, the Irish scientist Robert Boyle, with help from Robert Hooke, built an air pump. Using this pump, they discovered something important: if you keep the temperature the same, the pressure and volume of a gas are linked. When the volume goes down, the pressure goes up, and vice-versa (PV=constant).
At that time, people thought air was made of particles that didn't move. The idea that heat was caused by moving molecules came much later. Boyle's discovery was a big step towards understanding how gases behave.
Key Gas Laws
- Boyle's law (1662): Describes the relationship between pressure and volume of a gas.
- Charles's law (published 1802 by Joseph Louis Gay-Lussac, based on earlier work by Jacques Charles): Describes how gas volume changes with temperature.
- Gay-Lussac's law (1802): Describes how gas pressure changes with temperature.
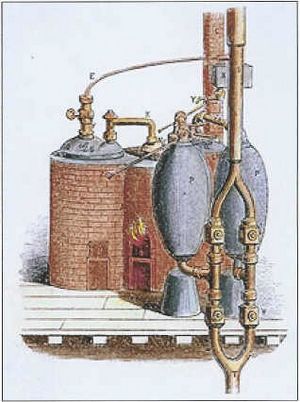
The Steam Digester
In 1679, Denis Papin, who worked with Boyle, invented a "bone digester." This was a sealed pot that trapped steam until it built up high pressure. Later versions had a special valve to release steam, stopping the pot from exploding. Papin watched this valve move up and down and got the idea for a piston and cylinder engine.
Although Papin didn't build a working engine, his ideas inspired Thomas Newcomen. In 1697, Newcomen greatly improved upon Thomas Savery's earlier "fire engine" by adding a piston. This made it much better for doing mechanical work, like pumping water out of mines. Many consider Newcomen's engine the first true steam engine.
Heat Transfer
We all know that hot air rises. Edmond Halley (the astronomer who discovered Halley's Comet) was the first to realize how important this was for understanding weather in 1686.
In 1701, Isaac Newton published his Newton's law of cooling, which describes how fast an object cools down.
The 1700s: New Ideas About Heat
Phlogiston Theory
In the 17th century, scientists believed in the "phlogiston theory." They thought that when things burned or metals rusted, they released a mysterious substance called phlogiston. This theory was later replaced by the "caloric theory" in the 18th century, marking a shift from old alchemy to modern chemistry.
Absolute Zero
In 1702, Guillaume Amontons introduced the idea of absolute zero. This is the lowest possible temperature where particles would theoretically stop moving. He based this idea on his observations of gases.
Kinetic Theory of Heat
One of the first scientists to suggest that heat was caused by tiny particles moving was Mikhail Lomonosov. He wrote: "Movement should not be denied based on the fact it is not seen... Just as in this case motion remains hidden due to perspective, it remains hidden in warm bodies due to the extremely small sizes of the moving particles."
Around the same time, Daniel Bernoulli published his book Hydrodynamics (1738). In it, he explained the pressure of a gas by thinking about its atoms hitting the walls of a container. He showed that this pressure was related to the average kinetic energy of the gas. Bernoulli's ideas were ahead of their time and didn't become widely accepted until much later.
Thermochemistry and Steam Engines
Heat Capacity
The Scottish chemist Joseph Black discovered in the 1750s that different materials could hold different amounts of heat. He called this "heat capacity." Today, we understand heat as the transfer of energy. Even so, we still use the term "heat capacity."
Better Steam Engines

Before 1698, horses were used to lift water out of flooded mines. The invention of the Savery engine and later the Newcomen engine and Watt engine changed this. These early engines were slow and not very efficient. They converted less than 2% of the fuel's energy into useful work. This meant a lot of coal had to be burned to get a little bit of work. This inefficiency led scientists to look for better ways to understand how engines work, leading to the science of thermodynamics.
Caloric Theory
In the mid-to-late 1700s, many scientists believed that heat was an invisible fluid called "caloric." They thought caloric flowed from hotter objects to colder ones, making them warm. However, the idea that heat was caused by moving particles (kinetic theory) eventually replaced the caloric theory. By the end of the 19th century, the caloric theory was mostly gone.
Calorimetry
Scientists like Joseph Black and Antoine Lavoisier made important progress in measuring heat changes accurately using a device called a calorimeter. This field became known as thermochemistry. The development of steam engines made it even more important to measure how much heat different types of coal produced. Lavoisier used an ice calorimeter to study heat changes during chemical reactions, building on Black's work on the latent heat of water (the heat needed to change water's state, like from ice to liquid).
Heat and Friction
In the 19th century, scientists stopped believing in the idea of caloric. A big challenge to the caloric theory came from Benjamin Thompson (also known as Count Rumford) in 1798. He showed that boring cast iron cannons produced a huge amount of heat, which he believed came from friction. His experiments were among the first to show that heat was not a substance but a form of motion.
Early 1800s: Modern Thermodynamics Begins
Sadi Carnot: The Father of Thermodynamics
Even though early steam engines were not very good, they caught the attention of top scientists. One of them was Nicolas Léonard Sadi Carnot, who is called the "father of thermodynamics." In 1824, he published a book called Reflections on the Motive Power of Fire. This book talked about heat, power, and how efficient engines could be. Many people see this book as the start of thermodynamics as a modern science. (The word "thermodynamics" itself wasn't used until 1854 by Lord Kelvin.)
Carnot defined "motive power" as the "useful effect" an engine could produce. He also gave us the first modern definition of "work": weight lifted through a height. Understanding this "useful effect" and how it relates to work is still at the heart of thermodynamics today.
Melloni and Radiant Heat
In 1831, Macedonio Melloni showed that radiant heat (heat that travels as waves, like from the sun) could be reflected, refracted (bent), and polarized (made to vibrate in a specific direction) just like light. This was an important discovery because it suggested that heat and light were similar.
Joule and the Mechanical Equivalent of Heat
From 1843 onwards, James Prescott Joule did many experiments that showed a clear link between heat and mechanical work. In 1843, he found the mechanical equivalent of heat. This means he showed how much mechanical energy (like from a falling weight) was needed to produce a certain amount of heat.
In his most famous experiment in 1845, Joule used a falling weight to spin a paddle-wheel in a barrel of water. The friction from the paddle-wheel warmed the water. This experiment helped prove the idea of the conservation of energy, which states that energy cannot be created or destroyed, only changed from one form to another. It also explained why heat can do work.
Absolute Zero and the Kelvin Scale
The idea of absolute zero was further developed in 1848 by Lord Kelvin. He proposed a temperature scale, now called the Kelvin scale, where 0 Kelvin is absolute zero. This scale is very important in science because it starts at the true zero point of temperature.
Late 1800s: Deeper Understanding
Entropy and the Second Law of Thermodynamics
Lord Kelvin's Idea of Heat Loss
In 1851, Lord Kelvin realized that in all natural processes, some useful heat is always lost. This idea was later explained more dramatically by Hermann von Helmholtz in 1854, leading to the concept of the "heat death of the universe," where all energy would eventually spread out evenly, and no more work could be done.
Rudolf Clausius and Entropy
In 1865, the physicist Rudolf Clausius gave a name to this "lost" or "wasted" heat: entropy (symbolized as S). He used this concept to create his famous statement of the second law of thermodynamics. This law basically says that in any natural process, the total entropy of a system and its surroundings will always increase. This means that things tend to move towards a state of greater disorder or randomness.
Statistical Thermodynamics
Heat is Average Kinetic Energy
In 1857, Clausius clearly stated for the first time that heat is the average kinetic energy of molecules. This was a big step in connecting the microscopic world of atoms to the macroscopic world of heat.
Maxwell and Boltzmann
Clausius's idea interested the Scottish physicist James Clerk Maxwell. In 1859, Maxwell developed a way to describe how the speeds of molecules in a gas are distributed, known as the Maxwell–Boltzmann distribution. The Austrian physicist Ludwig Boltzmann later expanded this idea.
Together, Clausius and Maxwell helped create a new field called statistical thermodynamics around 1871. This field uses statistics to study how large numbers of particles behave at thermodynamic equilibrium (when no changes are happening). It helps us understand average properties like temperature, pressure, and volume.
Boltzmann and Entropy
Boltzmann made many important contributions to kinetic theory. He connected the kinetic energy of particles to their "degrees of freedom" (the ways they can move). In 1875, Boltzmann gave a precise formula for entropy S based on the number of possible ways W that molecules could be arranged:
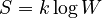
Here, k is the Boltzmann constant, a fundamental constant in physics. This formula showed that entropy is a measure of the disorder or randomness of a system.
Gibbs Free Energy
In 1876, the American chemical engineer Willard Gibbs published a paper where he introduced the Gibbs free energy equation. This equation helps measure the amount of "useful work" that can be obtained from chemical reactions. It's very important in chemistry and chemical engineering.
Enthalpy
Gibbs also came up with the idea we now call enthalpy (H). He called it "a heat function for constant pressure." The word "enthalpy" itself was coined much later, based on a Greek word meaning "to warm." Enthalpy is a measure of the total heat content of a system.
Stefan–Boltzmann Law
Maxwell's discovery in 1862 that light and radiant heat are both forms of electromagnetic waves led to more studies of thermal radiation. In 1879, Jožef Stefan observed that the total heat radiated from a perfect absorber (a "blackbody") is proportional to the fourth power of its temperature. This is known as the Stefan–Boltzmann law. Boltzmann later proved this law theoretically in 1884.
20th Century: Quantum and Beyond
Quantum Thermodynamics
In 1900, Max Planck found an accurate formula for the spectrum of black-body radiation. To make his formula work, he had to introduce a new idea: that energy comes in tiny, specific packets, or "quanta." This led to the discovery of Planck's constant and was a huge step in the development of quantum mechanics, which describes the behavior of matter and energy at the atomic and subatomic level. This idea also led to the field of quantum thermodynamics.
Third Law of Thermodynamics
In 1906, Walther Nernst stated the third law of thermodynamics. This law says that as a system approaches absolute zero, its entropy approaches a minimum constant value. This means that at absolute zero, a system would be in its most ordered state.
Expanding Thermodynamics
Building on these foundations, scientists like Lars Onsager, Erwin Schrödinger, and Ilya Prigogine applied the ideas of thermodynamics to almost every area of science, from biology to cosmology.
Branches of Thermodynamics
Thermodynamics has grown into many different fields over time:
- Thermochemistry – 1780s (how heat relates to chemical reactions)
- Classical thermodynamics – 1824 (the basic rules of heat and energy)
- Chemical thermodynamics – 1876 (how thermodynamics applies to chemistry)
- Statistical mechanics – around 1880s (using statistics to understand large groups of particles)
- Non-equilibrium thermodynamics – 1941 (studying systems that are not in balance)
- Biological thermodynamics – 1957 (how thermodynamics applies to living things)
- Quantum thermodynamics – 1968 (combining thermodynamics with quantum mechanics)
- Black hole thermodynamics – around 1970s (applying thermodynamic ideas to black holes)
- Atmospheric thermodynamics – around 1980s (how heat and energy work in the atmosphere)
Concepts from thermodynamics have also been used in other areas, like:
- Thermoeconomics – around 1970s (combining thermodynamics with economics)
Images for kids
See also
 In Spanish: Historia de la termodinámica para niños
In Spanish: Historia de la termodinámica para niños
- Conservation of energy: Historical development
- History of Chemistry
- History of Physics
- Thermodynamics
- Timeline of thermodynamics
- Vacuum