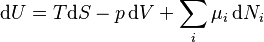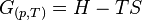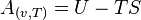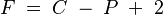Josiah Willard Gibbs facts for kids
Quick facts for kids
Josiah Willard Gibbs
|
|
|---|---|

Josiah Willard Gibbs
|
|
| Born | February 11, 1839 New Haven, Connecticut, U.S.
|
| Died | April 28, 1903 (aged 64) New Haven, Connecticut, U.S.
|
| Nationality | American |
| Alma mater | Yale College, Heidelberg University |
| Known for |
List
Statistical mechanics
Chemical thermodynamics Chemical potential Cross product Dyadics Exergy Principle of maximum work Phase rule Phase space Physical optics Physics of phase separation Statistical ensemble Surface stress Vector calculus Gibbs entropy Gibbs algorithm Gibbs distribution Gibbs' elasticity Gibbs function Gibbs free energy Gibbs' inequality Gibbs isotherm Gibbs' H theorem Gibbs lemma Gibbs measure Gibbs paradox Gibbs phenomenon Gibbs state Gibbs' thermodynamic surface Gibbs vector Gibbs–Appell equation of motion Gibbs–Donnan effect Gibbs–Duhem equation Gibbs–Helmholtz equation Gibbs–Marangoni effect Gibbs–Thomson equation Gibbs–Thomson effect Gibbs–Wulff theorem |
| Awards |
|
| Scientific career | |
| Fields | Chemistry · Mathematics · Physics |
| Institutions | Yale College |
| Thesis | On the form of the teeth of wheels in spur gearing (1863) |
| Doctoral advisor | Hubert Anson Newton |
| Doctoral students | Edwin Bidwell Wilson Irving Fisher Henry Andrews Bumstead Lynde Wheeler Lee De Forest |
| Influences | Rudolf Clausius James Clerk Maxwell Ludwig Boltzmann Jules Moutier |
| Influenced | Johannes Diderik van der Waals Pierre Duhem |
| Signature | |
Josiah Willard Gibbs (born February 11, 1839 – died April 28, 1903) was an American scientist. He made very important discoveries in physics, chemistry, and mathematics.
His work on thermodynamics helped turn physical chemistry into a serious science. Along with James Clerk Maxwell and Ludwig Boltzmann, he created statistical mechanics. This field explains how the rules of thermodynamics come from the behavior of many tiny particles. Gibbs also used Maxwell's equations to study physical optics, which is about how light behaves. As a mathematician, he developed modern vector calculus, a way to work with directions and forces.
In 1863, Yale gave Gibbs the first ever American doctorate in engineering. After spending three years studying in Europe, Gibbs worked at Yale for the rest of his life. He was a professor of mathematical physics from 1871 until he died in 1903. He worked mostly by himself but became the first American scientist to be famous around the world. Albert Einstein even called him "the greatest mind in American history." In 1901, Gibbs received the Copley Medal, a top science award, for his work in mathematical physics.
People have often noted how Gibbs lived a quiet life in New England. Yet, his ideas had a huge impact worldwide. Even though his work was mostly about theories, its practical value became clear later. This happened with the growth of industrial chemistry in the 1900s.
Contents
About Josiah Willard Gibbs
His Family and Early Life
Josiah Willard Gibbs was born in New Haven, Connecticut. His family was an old Yankee family with many famous American church leaders and professors. He was the fourth of five children and the only son. His father, Josiah Willard Gibbs Sr., was a linguist and theologian. He taught at Yale Divinity School.
His father is remembered for helping the African passengers of the ship La Amistad. He found someone who could translate for them. This allowed them to speak during their trial after they rebelled against being sold as slaves.
Willard Gibbs shared his name with his father and other family members. To avoid confusion, his father was called "Josiah" and the son was called "Willard."
His Education
Willard Gibbs went to the Hopkins School. He started Yale College in 1854 when he was 15. At Yale, he won awards for being excellent in mathematics and Latin. He graduated in 1858 as one of the top students. He stayed at Yale for graduate school at the Sheffield Scientific School. When he was 19, he joined the Connecticut Academy of Arts and Sciences. This group was mostly made up of Yale professors.
Gibbs's main helper and friend was probably Hubert Anson Newton. Newton was an astronomer and mathematician who studied meteors. He remained Gibbs's close friend throughout his life. When his father died in 1861, Gibbs inherited enough money to live comfortably.
Young Gibbs often got sick with lung problems. Doctors worried he might get tuberculosis, which had killed his mother. He also had astigmatism, an eye problem. Treatment for this was not well known then, so Gibbs had to figure out his own diagnosis and even grind his own lenses. He wore glasses mainly for reading. His health and eyesight likely explain why he did not join the American Civil War (1861–65). He stayed at Yale during the war.
In 1863, Gibbs earned the first PhD in engineering given in the US. His thesis was about the best way to design gears for wheels. Yale was the first US university to offer a PhD degree, and Gibbs's was only the fifth PhD ever given in the US.
His Career: Early Years (1863–1873)
After graduating, Gibbs worked as a tutor at Yale for three years. For two years, he taught Latin. In the third year, he taught "natural philosophy," which is what physics was called then. In 1866, he patented a design for a railway brake. He also gave a talk about how to make measurement units more logical.
After his tutoring job ended, Gibbs traveled to Europe with his sisters. They spent the winter of 1866–67 in Paris. There, Gibbs attended lectures by famous scientists like Joseph Liouville. He studied very hard and caught a bad cold. A doctor worried about tuberculosis and told him to rest. He and his sisters spent several months relaxing on the French Riviera until he fully recovered.
Next, Gibbs went to Berlin. He attended lectures by mathematicians Karl Weierstrass and Leopold Kronecker, and chemist Heinrich Gustav Magnus. In August 1867, his sister Julia married Addison Van Name, who had been Gibbs's classmate at Yale. The newly married couple went back to New Haven, but Gibbs and his sister Anna stayed in Germany. In Heidelberg, Gibbs learned from physicists Gustav Kirchhoff and Hermann von Helmholtz, and chemist Robert Bunsen. At that time, German scientists were leaders in natural sciences, especially chemistry and thermodynamics.
Gibbs returned to Yale in June 1869. For a short time, he taught French to engineering students. Around this time, he also worked on a new design for a governor for steam engines. This was his last big project in mechanical engineering. In 1871, he became a Professor of Mathematical Physics at Yale. This was the first such professorship in the United States. Gibbs had his own money and had not yet published any work, so he was hired without a salary. He only taught graduate students.
His Career: Major Discoveries (1873–1903)
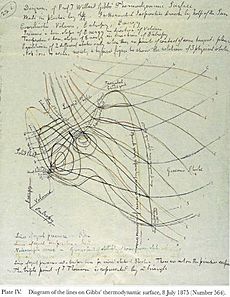
Gibbs published his first scientific work in 1873. His papers showed how to use geometry to represent thermodynamic ideas. He used different types of phase diagrams to help him think about his research. These were his favorite tools, much more than physical models. Even though few people understood his work, he sent copies to scientists in Europe. James Clerk Maxwell in Cambridge was very excited about it. Maxwell even made a clay model to show Gibbs's ideas. He sent one of the plaster copies to Gibbs, which is now at the Yale physics department.
Maxwell wrote about Gibbs's work in his book Theory of Heat in 1875. He also explained Gibbs's methods in a lecture in London. Sadly, Maxwell died young in 1879. People in New Haven joked that "only one man lived who could understand Gibbs's papers. That was Maxwell, and now he is dead."
Gibbs then looked at how his thermodynamic ideas could apply to chemical systems with many different parts. He wrote about this in a long paper called "On the Equilibrium of Heterogeneous Substances". It was published in two parts in 1875 and 1878. This work was about 300 pages long and had 700 math equations. It started with a quote about the first and second laws of thermodynamics: "The energy of the world is constant. The entropy of the world tends towards a maximum."
Gibbs's paper used his thermodynamic methods to explain many different physical and chemical events. It connected many facts and observations that seemed unrelated before. This work is seen as a cornerstone for physical chemistry. Wilhelm Ostwald, who translated Gibbs's paper into German, called Gibbs the "founder of chemical energetics."
Gibbs worked without pay until 1880. Then, Johns Hopkins University offered him a job with a salary. Yale responded by offering him $2,000 a year, which he accepted.
From 1880 to 1884, Gibbs worked on developing vector calculus. This was a new way to do math that was very useful for physicists. He created the ideas of the dot product and cross product for vectors. Another scientist, Oliver Heaviside, did similar work around the same time. Gibbs wanted other physicists to use his vector methods instead of the older quaternions. This led to some debates in the science journal Nature in the 1890s.
Gibbs's notes on vector calculus were printed for his students in 1881 and 1884. Later, his student Edwin Bidwell Wilson turned them into a textbook called Vector Analysis, published in 1901. This book helped make the "del" notation popular, which is still used today in electromagnetism and fluid mechanics. In other math work, he rediscovered the "Gibbs phenomenon" in Fourier series.
From 1882 to 1889, Gibbs wrote five papers on physical optics. He studied phenomena like birefringence (when light splits into two rays). He supported Maxwell's idea that light is an electromagnetic wave. Gibbs avoided guessing about the tiny structure of matter. He focused on problems that could be solved using general principles and facts proven by experiments. His methods were very original, and his results clearly showed that Maxwell's theory of light was correct.
Gibbs created the term statistical mechanics. He also introduced important ideas in this field, like chemical potential (1876) and statistical ensemble (1902). Gibbs explained the laws of thermodynamics using the statistical properties of systems with many particles. He wrote about this in his important textbook Elementary Principles in Statistical Mechanics, published in 1902, a year before he died.
Gibbs was a quiet person and focused intensely on his work. This made him less approachable to students. His main student was Edwin Bidwell Wilson, who said he saw little of Gibbs outside the classroom. Gibbs did supervise Irving Fisher's doctoral thesis on mathematical economics in 1891. After Gibbs died, Fisher paid to publish his Collected Works. Another famous student was Lee de Forest, who later helped invent radio technology.
Gibbs died in New Haven on April 28, 1903, at age 64. He had a severe intestinal blockage. His funeral was held two days later at his home. He was buried in the nearby Grove Street Cemetery. Yale held a memorial meeting for him in May. The famous British physicist J. J. Thomson attended and spoke.
His Personal Life
Gibbs never married. He lived his whole life in his childhood home with his sister Julia and her husband, Addison Van Name, who was the Yale librarian. Except for summer vacations in the Adirondack Mountains and White Mountains, his trip to Europe was almost the only time he spent outside New Haven. He joined Yale's College Church and attended regularly. He usually voted for the Republican candidate in presidential elections. However, he supported Grover Cleveland, a Democrat, in the 1884 election. This was because he was worried about political corruption. Not much else is known about his religious or political views, as he kept them private.
Gibbs did not write many personal letters, and many of them were lost. Besides his scientific papers, he only published two other things. One was a short tribute to Rudolf Clausius, a founder of thermodynamics. The other was a longer biography of his Yale mentor, H. A. Newton.
He was good with money and invested carefully. When he died in 1903, his estate was worth $100,000. For many years, he helped manage the Hopkins School, his old school. US President Chester A. Arthur appointed him to a conference of electricians in 1884, where Gibbs led one of the sessions. Gibbs was a skilled horseman and was often seen driving his sister's carriage in New Haven.
What Gibbs Discovered
Chemical and Electrochemical Thermodynamics
Gibbs's papers from the 1870s introduced the idea of describing a system's internal energy (U) using entropy (S), along with volume (V), pressure (p), and temperature (T). He also introduced the idea of chemical potential (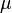 ). This is how much the energy changes when you add more molecules of a certain chemical.
). This is how much the energy changes when you add more molecules of a certain chemical.
He was the first to combine the first and second laws of thermodynamics. He showed how a tiny change in internal energy (dU) can be written as:
Here, T is the absolute temperature, p is the pressure, dS is a small change in entropy, and dV is a small change in volume. The last part adds up the chemical potential (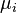 ) for each chemical multiplied by the small change in the number of moles (dNi).
) for each chemical multiplied by the small change in the number of moles (dNi).
Using this, he defined enthalpy (H) and Gibbs free energy (G).
This is similar to the Helmholtz free energy (A):
If the Gibbs free energy for a chemical reaction is negative, the reaction will happen on its own. If a chemical system is in equilibrium, the change in Gibbs free energy is zero.
His paper "On the Equilibrium of Heterogeneous Substances" (1874–78) was a major step in chemistry. In it, Gibbs created a strong mathematical theory for things like adsorption (when molecules stick to a surface), electrochemistry (chemistry with electricity), and the Marangoni effect (movement of fluid due to surface tension). He also created the phase rule:
This rule tells you how many variables (like temperature or pressure) you can control independently in a mixture. F is the number of variables, C is the number of components (different chemicals), and P is the number of phases (like solid, liquid, gas). The phase rule is very useful in fields like metallurgy (working with metals) and mineralogy (studying minerals).
Statistical Mechanics
Along with James Clerk Maxwell and Ludwig Boltzmann, Gibbs started "statistical mechanics." This is a field of physics that explains how systems behave based on the statistics of their many tiny particles. He introduced the idea of "phase of a mechanical system." He used this to define different types of "ensembles" (collections of possible states). This gave a more general way to understand the statistical properties of systems with many particles than Maxwell and Boltzmann had before him.
Gibbs expanded on Boltzmann's idea of entropy ( ). He defined the entropy of any collection of states as:
). He defined the entropy of any collection of states as:
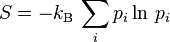 ,
,
where 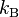 is the Boltzmann constant, and the sum is over all possible tiny states (
is the Boltzmann constant, and the sum is over all possible tiny states (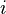 ), with
), with 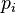 being the chance of that state happening. This formula later became very important in Claude Shannon's information theory.
being the chance of that state happening. This formula later became very important in Claude Shannon's information theory.
Henri Poincaré wrote in 1904 that Gibbs was the one who most clearly explained why large physical processes seem to go only one way (irreversibility). Gibbs's work on irreversibility and his ideas about the H-theorem and ergodic hypothesis greatly influenced 20th-century physics.
Gibbs knew that applying a certain theorem (the equipartition theorem) to large systems of classical particles didn't explain the measured specific heats of solids and gases. He said this showed the danger of basing thermodynamics on "guesses about what matter is made of." Gibbs's own system for statistical mechanics could still be used even after scientists discovered that tiny particles follow quantum rules, not the classical rules known in Gibbs's time. His solution to the "Gibbs paradox" (about the entropy of mixing gases) is now seen as an early hint that particles are indistinguishable in quantum physics.
Physical Optics

Gibbs's work on physical optics is less known today, but it was a big step forward for classical electromagnetism. He applied Maxwell's equations to explain how light behaves, such as birefringence (light splitting), dispersion (light separating into colors), and optical activity (light rotating as it passes through a substance).
In this work, Gibbs showed that Maxwell's equations could explain these things without needing special guesses about what matter is made of. He also pointed out that Maxwell's equations naturally explain why light doesn't have a "longitudinal" wave (a wave that vibrates in the direction it travels). This was important for understanding light.
Soon after, Heinrich Hertz proved that light is electromagnetic through his experiments in Germany.
How Gibbs Was Recognized
Gibbs worked at a time when there wasn't much strong theoretical science in the United States. His research was hard for his students and colleagues to understand. He didn't try to make his ideas simpler or more popular. His important work on thermodynamics was mostly published in the Transactions of the Connecticut Academy. This journal was not widely read in the US or Europe. When Gibbs sent his long paper on the equilibrium of different substances to the academy, some professors said they didn't understand it at all. But they helped raise money to pay for printing all the math symbols in the paper.
Even though Maxwell quickly accepted it, Gibbs's way of showing thermodynamic laws using graphs only became widely used in the mid-1900s.
However, Gibbs did receive major honors in the US. He was elected to the National Academy of Sciences in 1879. He also received the 1880 Rumford Prize for his work on chemical thermodynamics. He was given honorary doctorates (special degrees) by Princeton University and Williams College.
In Europe, Gibbs became an honorary member of the London Mathematical Society in 1892. He was elected a Foreign Member of the Royal Society in 1897. He was also elected to the science academies in Prussia and France. He received honorary doctorates from universities in Dublin, Erlangen, and Christiania (now Oslo). The Royal Society gave Gibbs the Copley Medal in 1901. This was considered the highest international award in natural sciences. They noted that he was "the first to apply the second law of thermodynamics to fully discuss the relationship between chemical, electrical and thermal energy." Gibbs stayed in New Haven, so a US naval officer accepted the award for him in London.
Mathematician Gian-Carlo Rota once found a handwritten list of over two hundred famous scientists of Gibbs's time, including Poincaré and Boltzmann. This showed that Gibbs's work was better known among top scientists than people might think from his publications. The fact that his main paper was translated into German (by Wilhelm Ostwald) and French (by Henri Louis Le Châtelier) also shows how important his work was in Europe.
How Gibbs Influenced Others
Gibbs's most direct influence was on physical chemistry and statistical mechanics. He greatly helped to create both of these fields. During his lifetime, his phase rule was proven true by experiments. It became widely used in many areas, including industrial chemistry.
When Dutch physicist Johannes Diderik van der Waals won the 1910 Nobel Prize for his work on gases and liquids, he said Gibbs's work had a great influence on him. Max Planck won the 1918 Nobel Prize for his work on quantum mechanics. His work was based a lot on the thermodynamics of Kirchhoff, Boltzmann, and Gibbs. Planck said that Gibbs's name "will ever be reckoned among the most renowned theoretical physicists of all times."
In the early 1900s, two important textbooks on chemical thermodynamics were published. Both used and expanded on Gibbs's work. These were Thermodynamics and the Free Energy of Chemical Processes (1923) by Gilbert N. Lewis and Modern Thermodynamics by the Methods of Willard Gibbs (1933) by Edward A. Guggenheim.
Gibbs's work on statistical ensembles, in his 1902 textbook, had a big impact on both theoretical physics and pure mathematics.
Albert Einstein wrote three papers on statistical mechanics between 1902 and 1904. He didn't know about Gibbs's work at first. After reading Gibbs's textbook (translated into German in 1905), Einstein said that Gibbs's approach was better than his own. He said he wouldn't have written his papers if he had known Gibbs's work.
Gibbs's early papers on using graphs in thermodynamics showed a very original understanding of what mathematicians later called "convex analysis." These ideas were not fully explored for about 75 years. Important math ideas based on Gibbs's work include the Gibbs lemma in game theory, the Gibbs inequality in information theory, and Gibbs sampling in computational statistics.
The development of vector calculus was Gibbs's other great math contribution. The 1901 textbook Vector Analysis by E. B. Wilson, based on Gibbs's lectures, helped spread the use of vector methods. It replaced the older "quaternions" that were common in science before.
At Yale, Gibbs also taught Lee De Forest, who invented the triode amplifier and is called the "father of radio." De Forest said Gibbs influenced him to realize that leaders in electrical development would be those who studied the "higher theory of waves and oscillations." Another student of Gibbs who helped develop radio technology was Lynde Wheeler.
Gibbs also indirectly influenced mathematical economics. He supervised the thesis of Irving Fisher, who got the first PhD in economics from Yale in 1891. Fisher compared Gibbs's idea of balance in physical systems to the balance of markets. He used Gibbs's vector notation. Gibbs's student Edwin Wilson later taught Paul Samuelson, a famous American economist and Nobel Prize winner. Samuelson said his understanding of prices came from "the great thermodynamicist, Willard Gibbs of Yale."
Mathematician Norbert Wiener called Gibbs's use of probability in statistical mechanics "the first great revolution of twentieth century physics." It greatly influenced his idea of cybernetics (the study of control and communication in machines and living things).
How Gibbs Is Remembered
When German physical chemist Walther Nernst visited Yale in 1906, he was surprised there was no memorial for Gibbs. Nernst donated his lecture fee to the university to help pay for a monument. This was a bronze plaque by sculptor Lee Lawrie, put up in 1912. In 1910, the American Chemical Society created the Willard Gibbs Award for important work in chemistry. In 1923, the American Mathematical Society started the Josiah Willard Gibbs Lectureship, to teach the public about math and its uses.
In 1945, Yale University created the J. Willard Gibbs Professorship in Theoretical Chemistry. Yale also built the Josiah Willard Gibbs Laboratories and created the J. Willard Gibbs Assistant Professorship in Mathematics. Yale has held two conferences about Gibbs's life and work. Rutgers University has a J. Willard Gibbs Professorship of Thermomechanics.
Gibbs was chosen for the Hall of Fame for Great Americans in 1950. The oceanographic research ship USNS Josiah Willard Gibbs (T-AGOR-1) was used by the United States Navy from 1958 to 1971. Gibbs crater on the Moon was named after him in 1964.
Edward A. Guggenheim started using the symbol G for the Gibbs free energy in 1933. This is now used everywhere. In 1960, some scientists suggested the name "gibbs" for the unit of entropy, but this name was not widely adopted.
In 1954, Albert Einstein was asked who the greatest thinkers he knew were. Einstein replied: "Hendrik Lorentz", adding "I never met Willard Gibbs; perhaps, had I done so, I might have placed him beside Lorentz." Author Bill Bryson called Gibbs "perhaps the most brilliant person that most people have never heard of."
In 1958, a ship called USS San Carlos was renamed USNS Josiah Willard Gibbs. It became an oceanographic research ship.
In Books and Art
In 1909, the American writer Henry Adams wrote an essay trying to apply Gibbs's phase rule to human history. People criticized this because Adams didn't fully understand the science.

In the 1930s, poet Muriel Rukeyser became very interested in Willard Gibbs. She wrote a long poem about him and a book-length biography called Willard Gibbs (1942).
In 1946, Fortune magazine featured Gibbs's thermodynamic surface on its cover. This was a model Maxwell built based on Gibbs's ideas. The magazine saw it as "the abstract creation of a great American scientist that lends itself to the symbolism of contemporary art forms."
Gibbs's nephew, Ralph Gibbs Van Name, a chemistry professor at Yale, was not happy with Rukeyser's biography. He felt she lacked scientific training. With his encouragement, physicist Lynde Wheeler published a new biography of Gibbs in 1951.
Gibbs and Rukeyser's biography are important in the poetry collection True North (1997) by Stephanie Strickland. In fiction, Gibbs appears as a teacher to a character in Thomas Pynchon's novel Against the Day (2006). This novel also talks about the splitting of light by Iceland spar, which Gibbs studied.
Gibbs Stamp (2005)
In 2005, the United States Postal Service released a series of stamps called American Scientists. One stamp featured Gibbs, along with John von Neumann, Barbara McClintock, and Richard Feynman. The first day of issue ceremony was held at Yale University.
Kenneth R. Jolls, a chemical engineering professor, helped design the Gibbs stamp. The stamp calls Gibbs a "thermodynamicist" and shows a diagram from Maxwell's 1875 book, which illustrates Gibbs's thermodynamic surface for water. Tiny writing on Gibbs's collar shows his original math equation for how a substance's energy changes.
Main Areas of His Work
- Physical chemistry: free energy, phase diagram, phase rule, transport phenomena
- Statistical mechanics: statistical ensemble, phase space, chemical potential, Gibbs entropy, Gibbs paradox
- Mathematics: Vector Analysis, convex analysis, Gibbs phenomenon
- Electromagnetism: Maxwell's equations, birefringence
Images for kids
See also
 In Spanish: Josiah Willard Gibbs para niños
In Spanish: Josiah Willard Gibbs para niños
- Concentration of measure in physics
- Thermodynamics of crystal growth
- Governor (device)
- List of notable textbooks in statistical mechanics
- List of theoretical physicists
- List of things named after Josiah W. Gibbs
- Timeline of United States discoveries
- Timeline of thermodynamics
 | Delilah Pierce |
 | Gordon Parks |
 | Augusta Savage |
 | Charles Ethan Porter |