Solution (chemistry) facts for kids

In chemistry, a solution is a special type of homogeneous mixture. This means it looks the same all the way through. It is made of two or more substances. In a solution, a solute is a substance that gets dissolved. The other substance, which does the dissolving, is called the solvent.
Imagine you put sugar in water. The sugar is the solute, and the water is the solvent. The water molecules pull the sugar particles apart. Then, the water molecules surround these tiny sugar particles. This makes the sugar spread out evenly in the water. This mixing happens at a very small level. The solution usually takes on the physical state of the solvent. For example, sugar water is a liquid, just like water.
An important part of a solution is its concentration. This tells you how much solute is in a certain amount of solution or solvent. When water is the solvent, we call it an "aqueous solution".
Contents
What Makes a Solution Special?
Solutions have some unique features:
- A solution is a homogeneous mixture. This means it looks uniform throughout.
- You cannot see the tiny particles of solute in a solution. In a suspension, you might see particles.
- Light beams do not scatter when they pass through a solution. This is different from a suspension, where particles can scatter light.
- Solutions are stable. The solute will not settle to the bottom. This happens unless you add too much solute. If you add too much, the extra will stay solid.
- You cannot separate the solute from a solution by filtration. This means you can't use a filter to get the dissolved sugar out of water.
- A solution has only one phase. This means it's all liquid, all gas, or all solid.
Different Kinds of Solutions
A mixture is homogeneous if its parts form a single phase. It is heterogeneous if its parts are in different phases. In a solution, the substance present in the largest amount is usually the solvent. Solvents can be gases, liquids, or solids. The solution will have the same physical state as the solvent.
Gases Mixing Together
When the solvent is a gas, only other gases can dissolve in it. A common example is air. Air is a solution of oxygen and other gases dissolved in nitrogen. Nitrogen makes up most of the air. Gas molecules are always moving. This movement helps gases mix completely. Gases do not separate into layers based on their weight.
Liquids Mixing Together
If the solvent is a liquid, many gases, liquids, and solids can dissolve in it. Here are some examples:
- Gas in liquid:
- Oxygen dissolved in water. Fish need this to breathe!
- Carbon dioxide in water. This is what makes carbonated water fizzy. The bubbles you see are gas escaping, not the dissolved gas itself.
- Liquid in liquid:
- Alcoholic drinks are solutions of ethanol (alcohol) in water.
- Solid in liquid:
- Sucrose (table sugar) dissolved in water.
- Sodium chloride (table salt) dissolved in water. This forms an electrolyte. When salt dissolves, it breaks into tiny charged particles called ions.
Solutions where water is the solvent are very common. They are called aqueous solutions. If the liquid solvent is not water, they are called non-aqueous solutions.
Some liquid mixtures are not solutions. For example, colloids, suspensions, and emulsions are not true solutions. They look different throughout.
Your body fluids are complex liquid solutions. They contain many dissolved substances. These include electrolytes like potassium ions. They also have molecules like sugar and urea. Oxygen and carbon dioxide are also important in your blood chemistry.
Solids Mixing Together
If the solvent is a solid, gases, liquids, and other solids can dissolve in it.
- Gas in solid:
- Liquid in solid:
- Solid in solid:
How Much Can Dissolve? (Solubility)
The ability of one substance to dissolve in another is called solubility. When two liquids can completely dissolve in each other, they are called miscible. For example, water and ethanol are miscible. If two substances can never mix to form a solution, they are immiscible. Oil and water are immiscible.
All solutions have a positive entropy of mixing. This means that mixing usually makes things more spread out and disordered, which is a natural process. However, there's a limit to how much solute can dissolve. At some point, no more solute can be dissolved. When this happens, the solution is called saturated.
The amount of solute that can dissolve can change. Factors like temperature and pressure affect solubility. Usually, a hotter solvent can dissolve more of a solid solute. Think about dissolving sugar in hot tea versus cold tea. However, most gases dissolve less as temperature increases.
How Solutions Change Things
When you add other substances to a compound, its physical properties can change. For example, its melting point or boiling point might be different. These changes are called colligative properties.
There are many ways to measure how much of one substance is dissolved in another. This is called concentration. Examples include molarity and volume fraction.
In an ideal solution, you can calculate its properties by combining the properties of its parts. If the solute and solvent are in equal amounts, it can be hard to say which is which. But usually, the one more often used as a solvent is still called the solvent.
Water and Other Liquid Solvents
All kinds of liquids can act as solvents. These include molten metals or molecular liquids. In chemistry, most solvents are molecular liquids. They can be grouped into polar and non-polar types. This depends on whether their molecules have a permanent electric dipole moment. Another way to group them is by whether they can form hydrogen bonds. These are called protic or aprotic solvents. Water is the most common solvent. It is both polar and can form hydrogen bonds.
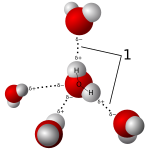
Salts dissolve well in polar solvents. This is because salts form positive and negative ions. These ions are attracted to the opposite charged ends of the solvent molecules. When salt dissolves in water, it's called hydration. The charged salt ions get surrounded by water molecules. These solutions are called electrolytes.
Polar solutes dissolve in polar solvents. They form polar bonds or hydrogen bonds. For example, all alcoholic drinks are aqueous solutions of ethanol. On the other hand, non-polar solutes dissolve better in non-polar solvents. Oil and grease are hydrocarbons. They mix easily with each other but not with water.
Think about an oil spill in the ocean. The oil does not dissolve in the water. Instead, it floats on the surface. This shows that oil and water are immiscible.
Making Solutions in the Lab
In science labs, people often make solutions from their ingredients. Chemists sometimes make strong stock solutions. These are very concentrated. They can then be diluted (made weaker) when needed for experiments. Standard solutions are solutions where the amount of solute is known very accurately.
See also
 In Spanish: Disolución para niños
In Spanish: Disolución para niños
- Molar solution
- Solubility equilibrium
- Total dissolved solids
- Upper critical solution temperature
- Lower critical solution temperature
 | Victor J. Glover |
 | Yvonne Cagle |
 | Jeanette Epps |
 | Bernard A. Harris Jr. |

