Ethanol facts for kids
Quick facts for kids Ethanol |
|
|---|---|
|
ethanol
|
|
| Other names | Absolute alcohol alcohol grain alcohol ethyl alcohol |
| Identifiers | |
| CAS number | |
| PubChem | |
| ChEBI | CHEBI:16236 |
| SMILES | CCO |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | Colorless liquid |
| Density | 0.7893 g/cm3 (at 20 °C) |
| Melting point | |
| Boiling point | |
| miscible | |
| Vapor pressure | 5.95 kPa (at 20 °C) |
| Refractive index (nD) | 1.3611 |
| Viscosity | 1.2 mPa·s (at 20 °C) |
| Hazards | |
| NFPA 704 |
|
| Flash point | 14 °C (Absolute) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |

Ethanol, also known as ethyl alcohol or grain alcohol, is a clear, colorless liquid that can easily catch fire. Its chemical formula is C2H5OH, which can also be written as C2H6O. Ethanol is a key part of many products and is used in various ways around the world. It is also a good solvent, meaning it can dissolve many other chemicals. Most of the ethanol people use is made by Yeast.
Contents
What is Ethanol Used For?
Medical Uses
As an Antiseptic
Ethanol is often used as an antiseptic to clean skin and surfaces. You can find it in medical wipes and hand sanitizers. It works by breaking down the outer layer of microorganisms and changing their proteins. This helps to kill most bacteria, fungi, and viruses. However, it does not work against tough bacterial spores.
As a Medicinal Solvent
Ethanol is used to dissolve many medicines that do not mix well with water. For example, some liquid pain medications, cough and cold medicines, and mouthwashes contain ethanol. It helps keep these medicines mixed and stable. It also acts as a preservative in many liquid medicines.
As an Antidote
In some serious cases, ethanol can be given as an antidote to help treat certain types of poisoning, such as from ethylene glycol or methanol.
Fuel Uses
Engine Fuel
The biggest use for ethanol is as a fuel for engines and as an additive to other fuels. Countries like Brazil use a lot of ethanol as engine fuel because they produce a lot of it. Gasoline sold in Brazil contains at least 25% ethanol. Special cars called "flex-fuel" cars can even run on pure ethanol or a mix of ethanol and gasoline.
In the United States, ethanol is often mixed with gasoline to create fuels like E10 (10% ethanol) and E85 (85% ethanol). Using ethanol as a fuel can help reduce harmful tailpipe emissions from cars. It can also improve engine performance because it has a higher octane rating than regular gasoline.
However, ethanol can also produce more formaldehyde and similar chemicals when it burns. These can contribute to ground level ozone and smog. Also, because ethanol mixes easily with water, it is not suitable for transport through long pipelines like other liquid fuels.
Rocket Fuel
Ethanol was an important fuel for early rockets. For example, the German V-2 rocket from World War II, which helped start the space age, used ethanol as its main fuel. After the war, the U.S. also used ethanol in rockets like the Redstone. While newer, more powerful fuels are now common, ethanol is still used in some lightweight rocket-powered aircraft.
Fuel Cells
Scientists are also looking into using ethanol in fuel cells. Fuel cells turn chemical energy directly into electricity. Ethanol is a good option because it is widely available, not too expensive, and not very toxic.
Home Heating and Cooking
Ethanol can be used in special fireplaces for heating homes or for decoration. It can also be used as a clean-burning fuel for cooking stoves.
Other Uses
Industrial Ingredient
Ethanol is a very important chemical in industry. It is used to make many other organic compounds, such as ethyl esters, acetic acid, and ethyl amines.
Solvent
Ethanol is known as a "universal solvent" because its unique structure allows it to dissolve both water-loving (hydrophilic) and water-hating (hydrophobic) substances. This makes it useful for extracting oils from plants.
You can find ethanol in paints, markers, and personal care products like mouthwashes, perfumes, and deodorants. It is also used in laboratories to help purify DNA and RNA.
Low-Temperature Liquid
Because ethanol has a very low freezing point (around -114°C or -173°F) and is not very toxic, it is used in laboratories as a cooling bath. This helps keep experiments at very cold temperatures. It is also the liquid used in alcohol thermometers.
How Ethanol Behaves Chemically
Chemical Formula and Structure
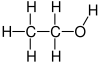
Ethanol is an alcohol with two carbon atoms. Its molecular formula is CH3CH2OH. This means it has a methyl group (CH3−) attached to a methylene group (−CH2–), which is then attached to a hydroxyl group (−OH). Sometimes, ethanol is simply called EtOH, where 'Et' stands for the ethyl group (C2H5−).
Physical Properties
Ethanol is a clear liquid that evaporates easily and has a mild smell. When it burns, it produces a blue flame that is often hard to see in normal light. The way ethanol behaves physically is mainly due to its hydroxyl group and its short carbon chain. The hydroxyl group allows ethanol molecules to form "hydrogen bonds" with each other. This makes ethanol thicker and less likely to evaporate than other similar chemicals that do not have this group.
Solvent Abilities
Ethanol is a very flexible solvent. It mixes well with water and many other organic solvents like acetone, benzene, and chloroform. It also mixes with light hydrocarbons and some chlorinated compounds.
Unlike longer alcohols, ethanol mixes completely with water. When ethanol and water are mixed, the total volume is slightly less than the sum of their individual volumes, and the mixing process releases a small amount of heat.
Pure ethanol can easily absorb water from the air because of its polar hydroxyl group. This polar nature also helps ethanol dissolve many ionic compounds, like sodium hydroxide and calcium chloride. Since ethanol also has a nonpolar part, it can dissolve nonpolar substances too, such as most essential oils and many flavorings and colors.
Adding even a small amount of ethanol to water greatly reduces the water's surface tension. This is why you might see "tears" or "legs" forming on the inside of a wine glass when you swirl it.
Mixing with Water (Azeotrope)
When ethanol and water are mixed, they form a special kind of mixture called an azeotrope. At normal air pressure, this mixture is about 95.6% ethanol by mass and boils at 78.1°C. This means you cannot get pure ethanol by simply boiling an ethanol-water mixture at normal pressure. However, at very low pressures, it is possible to get pure ethanol through distillation.
Flammability
Ethanol is a flammable liquid. An ethanol-water solution will catch fire if it is heated above a certain temperature called its flash point and then exposed to a flame. For example, a solution with 20% ethanol will catch fire at about 25°C (77°F). Pure ethanol has a flash point of about 13°C (55°F). This means ethanol mixtures can ignite even below average room temperature. Ethanol is considered a hazardous material if its concentration is above 2.35% by mass.
Dishes that use burning alcohol for cooking effects are called flambé.
Where is Ethanol Found Naturally?
Ethanol is naturally produced by yeast as a byproduct of their metabolism. This means it can be found wherever yeast lives, such as in overripe fruit. It is also produced by some plants during germination, especially when there isn't much oxygen.
Ethanol has even been found in outer space, forming an icy layer around dust particles in large clouds between stars. Small amounts of ethanol are also naturally present in the breath of healthy people. There is a rare medical condition called "auto-brewery syndrome" where a person's own digestive system produces intoxicating amounts of ethanol through fermentation.
Images for kids
-
Ethanol pump station in São Paulo, Brazil
-
A Ford Taurus fueled by ethanol in New York City
-
U.S. Postal Service truck running on E85 in Minnesota
See also
 In Spanish: Etanol para niños
In Spanish: Etanol para niños