History of biochemistry facts for kids
Biochemistry is a science that looks at the chemical processes happening inside living things. It helps us understand how our bodies work, from the smallest cells to how we grow and use energy. While people have been curious about life for a very long time, biochemistry as a specific science really started around the early 1800s.
Some scientists think biochemistry began in 1833 when Anselme Payen discovered the first enzyme called diastase (now known as amylase). Others point to Eduard Buchner's work in 1897, when he showed that a complex process like alcoholic fermentation could happen outside of living cells. Another important person was Justus von Liebig, who wrote about the chemistry of how bodies use food in 1842. Even earlier, in the 1700s, Antoine Lavoisier studied how living things breathe and how food breaks down.
The word biochemistry comes from two parts: bio- meaning 'life', and chemistry. It was first used in English in 1848. In 1877, Felix Hoppe-Seyler used the German word Biochemie to mean the same thing as "physiological chemistry" and suggested setting up special places to study it. However, many people say German chemist Carl Neuberg officially created the term for this new science in 1903.
Biochemistry studies the amazing chemical reactions that keep living things alive. It explores the parts of cells, like proteins, carbohydrates, lipids, and nucleic acids. It also looks at how our bodies get and use energy through metabolism, and how cells communicate using chemical signals. In the last 40 years, biochemistry has helped explain so much about life that almost all areas of biology and medicine now use biochemical research.
Many important molecules in living things are large and complex. These are called polymers, and they are made of smaller, repeating units called monomers. For example, proteins are polymers made from smaller units called amino acids. Carbohydrates are made from sugars. Lipids are built from fatty acids and glycerols. And nucleic acids, like DNA, are made from nucleotides. Biochemistry helps us understand how these molecules work, especially how enzymes speed up chemical reactions. It also explains how cells get energy and how hormones work in our bodies. Other areas include understanding the genetic code (DNA and RNA), how proteins are made, how things move in and out of cells, and how cells send messages to each other.
Contents
Early Ideas About Life's Chemistry
You could say that the study of biochemistry began a very long time ago, when people first became curious about how living things work. For example, the ancient Chinese developed a system of medicine based on ideas like yin and yang and the five phases. These ideas came from their interest in both chemistry (alchemy) and biology.
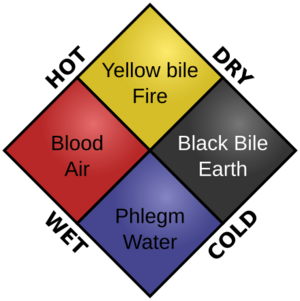
In ancient India, people were interested in medicine and thought bodies were made of different tissues. They also had a concept of three "humors," similar to the ancient Greeks' idea of four humors. The Greeks believed that good health came from a balance of these four humors and the four elements in the body.
Later, the Islamic world made big steps in early biology and chemistry. For example, Avicenna introduced the idea of clinical trials and clinical pharmacology in his book The Canon of Medicine. In chemistry, early progress came from exploring alchemy, but also from studying metallurgy (working with metals), developing the scientific method, and early ideas about atomism (that everything is made of tiny particles). More recently, important discoveries in chemistry include Dmitri Mendeleev's periodic table, John Dalton's atomic model, and the idea of the conservation of mass. This last idea is very important because it connects chemistry with how energy works (thermodynamics).
Enzymes: The Body's Helpers
Even in the late 1700s and early 1800s, people knew that stomach juices could digest meat and that plant extracts could turn starch into sugars. But they didn't know how these things happened.
In the 1800s, Louis Pasteur studied how yeast turns sugar into alcohol (fermentation). He thought this process was caused by a "vital force" inside the yeast cells, which he called ferments. He believed these ferments only worked inside living organisms.
Then, in 1833, Anselme Payen discovered the first enzyme, which he named diastase. Later, in 1878, a German scientist named Wilhelm Kühne came up with the word enzyme. This word comes from a Greek word meaning 'in leaven' (like yeast).
A big breakthrough happened in 1897 when Eduard Buchner showed that yeast extracts could ferment sugar even without living yeast cells. He found that the sugar still changed, and he named the enzyme responsible for this zymase. In 1907, he won the Nobel Prize in Chemistry for this discovery. After Buchner's work, enzymes are usually named after what they act on, adding -ase to the end. For example, lactase breaks down lactose.
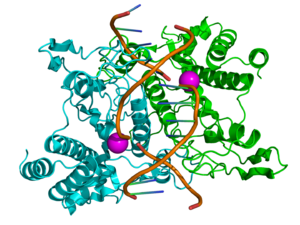
Once scientists knew that enzymes could work outside living cells, the next step was to figure out what they were made of. Many thought enzymes were connected to proteins, but some, like Richard Willstätter, argued that proteins just carried the real enzymes. However, in 1926, James B. Sumner proved that the enzyme urease was a pure protein and even managed to make it into crystals. He did the same for catalase in 1937. The idea that pure proteins could be enzymes was fully confirmed by John Howard Northrop and Wendell Meredith Stanley, who worked on digestive enzymes like pepsin. These three scientists shared the 1946 Nobel Prize in Chemistry.
Being able to crystallize enzymes meant scientists could use x-ray crystallography to see their exact structures. The first enzyme structure to be fully mapped out was lysozyme, which is found in tears and egg whites and helps break down bacteria. This was done by a team led by David Chilton Phillips in 1965. This detailed look at lysozyme started a new field called structural biology, helping us understand exactly how enzymes work at a tiny, atomic level.
Metabolism: How Bodies Use Energy
Early Ideas About Metabolism

The word metabolism comes from a Greek word meaning 'change'. People have been studying metabolism for over 800 years. The earliest known metabolic studies began in the 1200s with a Muslim scholar named Ibn al-Nafis. He wrote that "the body and all its parts are always breaking down and being rebuilt, so they are always changing."
While al-Nafis was the first doctor to show interest in these ideas, the first controlled experiments on human metabolism were published by Santorio Santorio in 1614. In his book, he described how he weighed himself before and after eating, sleeping, and working. He found that most of the food he ate was lost through what he called "insensible perspiration" (like sweat and breath).
Metabolism in the 20th Century
One of the most important biochemists in modern times was Hans Adolf Krebs. He made huge contributions to understanding metabolism. Krebs discovered the urea cycle and, later, working with Hans Kornberg, the citric acid cycle (also known as the Krebs cycle) and the glyoxylate cycle. These discoveries earned Krebs the Nobel Prize in physiology in 1953, which he shared with Fritz Albert Lipmann, who helped discover coenzyme A, an important molecule in energy production.
How Glucose is Absorbed
In 1960, biochemist Robert K. Crane discovered how our bodies absorb glucose (a type of sugar) from our intestines. He found that glucose is absorbed along with sodium, in a process called cotransport. This was a revolutionary idea in biology. This discovery wouldn't have been possible without the earlier work of German chemist Hermann Emil Fischer, who figured out the structure of glucose nearly 60 years before and won a Nobel Prize for it.
Glycolysis: Energy from Sugar
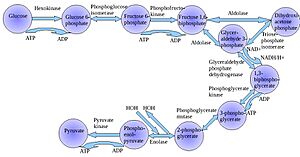
Metabolism involves breaking down molecules (catabolism) and building larger ones (anabolism). A key part of this is how our bodies use glucose to make adenosine triphosphate (ATP), which is the main energy currency of cells.
The most common way our bodies break down glucose is through a process called glycolysis, following the Embden-Meyerhof-Parnas (EMP) Pathway. This pathway was discovered by Gustav Embden, Otto Meyerhof, and Jakob Karol Parnas. They showed that glycolysis is very important for how efficiently our bodies produce energy. By understanding each step in this process, doctors can find problems in metabolism, like pyruvate kinase deficiency, which can lead to serious anemia. This is crucial because cells, and therefore living things, cannot survive without properly working metabolic pathways.
New Tools for Biochemistry (20th Century)
Biochemistry has grown a lot since the mid-1900s, thanks to new technologies. These include chromatography (separating mixtures), X-ray diffraction (seeing molecule structures), NMR spectroscopy (studying molecules using magnets), radioisotopic labelling (tracking molecules), electron microscopy (seeing tiny details), and molecular dynamics simulations (computer models of molecules).
These tools allowed scientists to discover and study many molecules and metabolic pathways inside cells, like glycolysis and the Krebs cycle. Some of these instruments, like the HWB-NMR shown here, can be very large and expensive, costing millions of dollars.
Polymerase Chain Reaction (PCR)
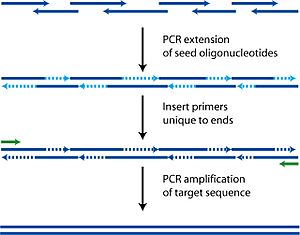
Polymerase chain reaction (PCR) is a technique that has changed modern biochemistry. It was developed by Kary Mullis in 1983. PCR allows scientists to make many copies of a specific piece of DNA. There are four main steps: 1) heating DNA to separate its strands (denaturation), 2) adding short DNA pieces (primers) that attach to the target DNA (annealing), 3) building new DNA strands (extension), and 4) repeating these steps to make millions of copies (amplification).
This technique is super important for biochemists who work with bacteria and gene expression (how genes are used). PCR is also used in labs to help diagnose diseases like some types of lymphomas and leukemia. Without PCR, many advancements in studying bacteria and proteins would not have happened. The invention of the thermal cycler, the machine that performs PCR, is just as important as the idea behind PCR itself. This shows how much technology helps science move forward.
See also
 In Spanish: Historia de la bioquímica para niños
In Spanish: Historia de la bioquímica para niños
- Agricultural chemistry § History
- History of biology
- History of chemistry
- History of molecular biology
- History of chromatography
- History of RNA biology
- Metabolism
- Citric acid cycle
 | Claudette Colvin |
 | Myrlie Evers-Williams |
 | Alberta Odell Jones |