Steelmaking facts for kids
Steelmaking is how we make steel from iron ore (rock with iron in it) or scrap metal. Think of it like cooking: we take raw ingredients and change them. In steelmaking, we remove things we don't want, like extra carbon, nitrogen, and sulfur. Then, we add special ingredients like manganese or chromium to make different kinds of steel.
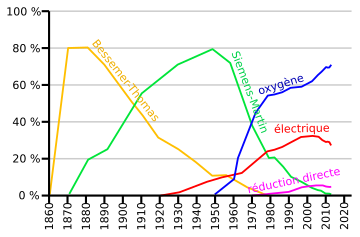
People have been making steel for thousands of years. But it wasn't until the mid-19th century that we learned to make it in huge amounts. In the 1850s and 1860s, new ways like the Bessemer process changed steelmaking into a big heavy industry.
Today, there are two main ways to make steel. One is called basic oxygen steelmaking. It uses liquid iron from a blast furnace and some scrap metal. The other is electric arc furnace (EAF) steelmaking. This method mostly uses scrap metal or a material called direct reduced iron (DRI). Basic oxygen steelmaking uses heat from chemical reactions. EAF steelmaking uses electricity to melt the metal.
Making steel is one of the industries that creates a lot of carbon pollution. As of 2020, steelmaking causes about 10% of all greenhouse gas emissions worldwide. To help stop global warming, the steel industry needs to find ways to pollute less. Scientists are looking into new ideas like capturing carbon pollution or using solar energy and wind energy to power the factories. They are also exploring using hydrogen as a cleaner fuel.
Contents
How did steelmaking start?

Steelmaking has been super important throughout history. It helped ancient, medieval, and modern societies grow. Early steelmaking methods were used in places like Ancient Iran, Ancient China, Ancient India, and Ancient Rome.
Cast iron is hard and breaks easily. But steel is strong, can be shaped, and is very useful. For most of history, people only made small amounts of steel. Then, in the 19th century, the Bessemer process was invented in Britain. This led to making steel in huge amounts. Now, mass-produced steel is a big part of the world's economy. It shows how advanced a country's technology is. The very first way to make steel was in a simple oven called a bloomery.
Early ways of making steel were often hard work and needed special skills. Some of these methods included:
- The finery forge, where a German method could make steel.
- Blister steel and crucible steel processes.
A big part of the Industrial Revolution was finding ways to make lots of metal that could be shaped. The puddling furnace was first used for wrought iron. Later, it was used for steel too.
The real change in modern steelmaking began in the late 1850s. That's when the Bessemer process became the first successful way to make a lot of steel. The open hearth furnace followed soon after.
How is steel made today?
Modern steelmaking has three main steps: primary, secondary, and tertiary.
- Primary steelmaking is when iron is first turned into steel.
- Secondary steelmaking is about adding or taking out other elements. This includes things like special metals (alloying agents) and gases.
- Tertiary steelmaking is the final step. Here, the steel is shaped into sheets, rolls, or other forms.
Many different methods can be used for each step.
Primary steelmaking
This is where the main work of turning iron into steel happens.
Basic oxygen steelmaking
Basic oxygen steelmaking is a way to turn pig iron (iron with lots of carbon) into steel. Workers blow oxygen through the hot, melted pig iron. This oxygen makes some of the carbon in the iron turn into gases like carbon monoxide and carbon dioxide. This process changes the iron into steel. Special materials called refractories line the inside of the furnace. These materials can handle the very high heat and the strong chemicals of the melted metal. The process is carefully controlled to remove unwanted things like silicon and phosphorus.
This modern method was created in 1948 by Robert Durrer. It improved on the older Bessemer converter by using pure oxygen instead of air. This made factories cheaper to build and made steel faster. It also meant fewer workers were needed. In 2013, about 70% of all steel made worldwide used this method. These furnaces can turn up to 350 tons of iron into steel in less than 40 minutes! This is much faster than the 10-12 hours it took with older methods.
Electric arc steelmaking
Electric arc furnace steelmaking makes steel from scrap metal or direct reduced iron. Powerful electric arcs melt these materials. In an electric arc furnace, a batch of iron is put into the furnace. Sometimes, some melted steel from the last batch is left inside. Gas burners can also help with the melting. Just like in basic oxygen steelmaking, special powders called fluxes are added. These protect the furnace lining and help remove unwanted stuff. Electric arc furnaces usually hold about 100 tons of metal. They can make steel in about 40 to 50 minutes. This method also lets workers add more special metals to create different steel types.
HIsarna process
The HIsarna ironmaking process turns iron ore almost directly into liquid iron. This process uses a special kind of blast furnace called a cyclone converter furnace. It means they don't have to make pig iron pellets first. Because it skips this step, the HIsarna process uses less energy. It also creates less carbon pollution than older steelmaking methods.
Hydrogen reduction
Steel can also be made from direct-reduced iron. This iron is made from iron ore using hydrogen gas. When hydrogen is used, steel can be made without burning fossil fuels. In 2021, a test factory in Sweden tried this method. The iron is then mixed with carbon in an electric arc furnace. Making hydrogen for this process needs a lot of electricity. It's estimated to cost 20-30% more than traditional ways. However, the cost of carbon pollution is rising. Experts think the hydrogen method could become cheaper than traditional methods by the 2030s.
Secondary steelmaking
Secondary steelmaking usually happens in large buckets called ladles. In these ladles, workers do many things to the steel. They remove oxygen, take out gases using a vacuum, add special metals, and clean up impurities. They also change the chemistry of the impurities and remove sulfur. Sometimes, they stir the steel with gas and heat it with electric arcs from the lid. Carefully controlling this step helps make very high-quality steel. This steel has very exact chemical makeup and is consistent.
Steelmaking and carbon pollution
As of 2021, making steel causes about 11% of the world's carbon dioxide pollution. It's also responsible for about 7% of all greenhouse gas emissions. For every ton of steel made, about 1.8 tons of carbon dioxide are released. Most of this pollution comes from the industrial process. In this process, coal is used to remove oxygen from iron ore in a blast furnace.
Here's the main chemical reaction that happens: Fe2O3(s) + 3 CO(g) → 2 Fe(s) + 3 CO2(g)
More carbon dioxide pollution comes from mining, cleaning, and shipping the iron ore. It also comes from basic oxygen steelmaking, calcination, and the hot blast process. Scientists are looking at ways to capture and use carbon pollution. They are also trying to use green hydrogen instead of carbon to reduce iron ore.
Mining and getting the ore
Mining coal and iron ore uses a lot of energy. It also causes many environmental problems. These include pollution, loss of plants and animals, cutting down forests, and greenhouse gas emissions. Iron ore is often shipped long distances to steel mills.
The blast furnace
To make pure steel, you need iron and carbon. Iron by itself isn't very strong. But adding a small amount of carbon (less than 1%) makes steel strong. The carbon in steel comes from coal, and the iron comes from iron ore. Iron ore is a mix of iron, oxygen, and other tiny bits. To make steel, the iron needs to be separated from the oxygen. A tiny bit of carbon also needs to be added. Both of these happen by melting the iron ore at very high temperatures (over 1,700 degrees Celsius). This is done with oxygen (from the air) and a type of coal called coke. At these high temperatures, the iron ore releases its oxygen. The carbon from the coke then carries this oxygen away as carbon dioxide gas.
Fe2O3(s) + 3 CO(g) → 2 Fe(s) + 3 CO2(g)
This reaction happens because carbon dioxide is a more stable form than iron oxide. The high temperatures give the energy needed for the reaction to start. A small amount of carbon sticks to the iron, forming pig iron. Pig iron is an in-between step before steel. It has too much carbon, about 4%.
Removing carbon
To lower the carbon in pig iron and get the right amount for steel, the pig iron is melted again. Then, oxygen is blown through it in a process called basic oxygen steelmaking. This happens in a large ladle. In this step, the oxygen connects with the extra carbon. It carries it away as carbon dioxide gas, which is another source of pollution. After this, the carbon content in the pig iron is low enough, and you have steel.
Calcination
More carbon dioxide pollution comes from using limestone. Limestone is melted at high temperatures in a reaction called calcination.
CaCO3(s) → CaO(s) + CO2(g)
Carbon dioxide is released in this reaction. Modern factories now use calcium oxide (CaO) instead. This material helps remove impurities like Sulfur or Phosphorus. It forms a waste product called slag and keeps carbon dioxide pollution low. For example, calcium oxide can react to remove silicon oxide impurities:
SiO2 + CaO → CaSiO3
Limestone is used to help clean the metal in both the blast furnace (to make pig iron) and in basic oxygen steelmaking (to make steel).
Hot blast
Even more carbon dioxide pollution comes from the hot blast. This is used to make the blast furnace even hotter. The hot blast blows hot air into the blast furnace. This helps the iron ore turn into pig iron by giving it the high energy it needs. The hot air can be from 900°C to 1300°C (1600°F to 2300°F). Sometimes, Oil, tar, natural gas, powdered coal, and oxygen are also put into the furnace. These combine with the coke to release more energy. This also increases the amount of reducing gases, making the process faster. If the air in the hot blast is heated by burning fossil fuels, which is often the case, this adds more carbon dioxide pollution.
How to reduce carbon pollution
There are many ways to cut down carbon pollution in the steel industry. These depend on how the steel is made. The most common way now is the blast furnace/basic oxygen furnace (BF/BOF) process. Ideas to reduce pollution fall into three main groups:
- Changing energy sources from fossil fuels to clean energy.
- Making the steelmaking process more efficient.
- Using new, innovative technologies.
Many of the new technologies are still being tested.
Using clean energy
The amount of carbon dioxide pollution changes with the energy source. If sustainable energy like wind or solar power is used, pollution can drop a lot. This can happen if these energies power electric arc furnaces or create hydrogen fuel. Companies in Europe are working on these ideas.
Capturing top gas
Top gas is the gas that usually leaves the blast furnace during steelmaking. This gas contains CO2. But it also has useful gases like H2 and CO. This top gas can be captured, the CO2 removed, and the useful gases put back into the blast furnace.
One study says this can cut blast furnace CO2 pollution by 75%. Another study says it's reduced by 56.5% if carbon is captured and stored. If only the useful gases are recycled, pollution is cut by 26.2%. To keep the captured carbon out of the air, it needs to be stored or used in some way.
Another idea is to use the top gas in a special turbine to make electricity. This electricity could then power the steelmaking process, especially if electric arc melting is used. Carbon could also be captured from gases in the coke oven. Right now, separating CO2 from other gases and the high cost of new equipment make this hard. But it could cut pollution by 65% to 80%.
Using scrap metal
Scrap metal in steelmaking means old steel that is no longer used. It also includes steel bits left over from making new parts. Steel is easy to separate and recycle because it's magnetic. Using scrap metal avoids about 1.5 tons of CO2 pollution for every ton of scrap used. Today, a lot of steel is recycled. All the scrap metal collected is used again in the steel industry.
Adding hydrogen to blast furnaces
In the blast furnace, iron oxides are changed by CO, H2, and carbon. Only about 10% of the iron oxides are changed by H2. By adding more H2, a bigger part of the iron oxides can be changed by hydrogen. This means less carbon is used and less CO2 is released. This process could cut pollution by about 20%.
The HIsarna process
The HIsarna ironmaking process makes iron in a cyclone converter furnace. It doesn't need the steps of choking or agglomeration first. This process reduces CO2 pollution by about 20%.
Hydrogen plasma
One new idea is to use hydrogen plasma technology. This would use hydrogen to change the iron oxides instead of CO or carbon. It would also melt the iron at very high temperatures. This project is still being developed.
Iron ore electrolysis
Another new technology is iron ore electrolysis. Here, electricity (electrons) is used to change the iron ore instead of H2, CO, or carbon. One way to do this is molten oxide electrolysis. In this method, the iron ore is heated and changed into iron and oxygen. A company called Boston Metal is testing this process. They plan to start making it commercially by 2026. They are expanding a test factory and building a production facility in Brazil.
Using biomass
In steelmaking, coal and coke are used for fuel and to change the iron. Biomass like charcoal or wood pellets could be used instead. However, burning biomass still releases carbon. It's more like a "carbon offset" than a real reduction. This means the pollution is "balanced" by the plants that grew the biomass. It's estimated to "offset" 5% to 28% of current CO2 pollution.
However, many people don't think "offsetting" is a good solution. Cutting down trees to make pellets or charcoal stops the trees from naturally taking in carbon. So, it's not truly reducing pollution.
What's next?
There are many new ways to reduce CO2 pollution in steelmaking. Some, like capturing top gas and using hydrogen in DRI/EAF, can be done now with existing factories. Others, like hydrogen plasma and iron ore electrolysis, are still being researched or tested. Despite these efforts, pollution from steelmaking was not falling in 2023.
See also
- Argon oxygen decarburization
- Basic oxygen steelmaking
- Blast furnace
- Calcination
- Carbon additive
- Decarburization
- FINEX
- Flodin process
- History of the steel industry (1850–1970)
- History of the steel industry (1970–present)
- Metallurgical coal
- Steel mill
 | Bayard Rustin |
 | Jeannette Carter |
 | Jeremiah A. Brown |