William Lipscomb facts for kids
Quick facts for kids
William N. Lipscomb Jr.
|
|
|---|---|
 |
|
| Born |
William Nunn Lipscomb Jr.
December 9, 1919 |
| Died | April 14, 2011 (aged 91) |
| Nationality | American |
| Alma mater | University of Kentucky California Institute of Technology |
| Spouse(s) |
Mary Adele Sargent
(m. 1944; div. 1983)Jean Evans
(m. 1983) |
| Children | 4 |
| Awards | Peter Debye Award (1973) Nobel Prize in Chemistry (1976) |
| Scientific career | |
| Fields | Nuclear magnetic resonance Theoretical chemistry Boron chemistry Biochemistry |
| Institutions | University of Minnesota Harvard University |
| Thesis | Part 1: Electron diffraction investigations of vanadium tetrachloride, dimethylketene dimer, tetrachloroethylene, and trichloroethylene Part 2: The Crystal Structure of Methylammonium Chloride (1946) |
| Doctoral advisor | Linus Pauling |
| Doctoral students |
|
| Other notable students | Martha L. Ludwig Michael Rossmann Raymond C. Stevens |
William Nunn Lipscomb Jr. (December 9, 1919 – April 14, 2011) was an American chemist. He won the Nobel Prize in Chemistry in 1976. Lipscomb studied nuclear magnetic resonance, theoretical chemistry, boron chemistry, and biochemistry.
Contents
Biography
Early life and education
William Lipscomb was born in Cleveland, Ohio, in 1919. His family moved to Lexington, Kentucky when he was young. He lived there until he finished college. In 1941, he earned his Bachelor of Science degree in Chemistry from the University of Kentucky.
He then went on to get his Doctor of Philosophy degree in Chemistry. He studied at the California Institute of Technology (Caltech). He finished his Ph.D. in 1946.
From 1946 to 1959, Lipscomb taught at the University of Minnesota. After that, he became a professor of chemistry at Harvard University in 1959. He taught there until 1990, becoming a professor emeritus.
Lipscomb was married to Mary Adele Sargent from 1944 to 1983. They had three children. He later married Jean Evans in 1983, and they adopted one daughter. He lived in Cambridge, Massachusetts, until he passed away in 2011 from pneumonia.
Growing up with science
Lipscomb's family encouraged him to be responsible and independent. His mother taught music, and his father was a doctor.
As a child, Lipscomb loved collecting things. He collected animals, insects, rocks, and minerals. He also became interested in astronomy. He visited the Observatory at the University of Kentucky. A professor there, H. H. Downing, gave him a book about astronomy. Lipscomb learned a lot about physics from this book and from talking to Downing.
He also worked on other projects with friends. They built Morse-coded message systems and crystal radio sets. Many of these friends later became scientists or doctors.
When he was 12, Lipscomb received a small Gilbert chemistry set. He expanded it by ordering more chemicals and equipment. He even used his father's doctor's discount to buy chemicals. He made his own fireworks and entertained guests with chemical reactions. His mother only questioned his hobby once. That was when he tried to get a lot of urea from urine.
Lipscomb's father had many large medical books. Reading these books helped Lipscomb later decide to study biochemistry. If he had become a doctor, he would have been the fourth doctor in his family line.
High school and college studies
Lipscomb's high school chemistry teacher, Frederick Jones, gave him college chemistry books. He only asked Lipscomb to take the exams. While others were in class, Lipscomb did his own research. He tried to make hydrogen from sodium formate and sodium hydroxide. He thought his work was new, but later found out it had been done before.
He also took a high school physics course. He won first prize in the state contest for physics. He became very interested in special relativity.
At the University of Kentucky, Lipscomb had a music scholarship. But he spent a lot of time studying science on his own. He read books on quantum mechanics and chemical bonding. One of his professors, Robert H. Baker, suggested a research project. This led to Lipscomb's first published scientific paper.
For graduate school, Lipscomb chose Caltech. He turned down offers from other universities. At Caltech, he first planned to study theoretical quantum mechanics. But he soon switched to the Chemistry Department. This was because of the influence of Professor Linus Pauling. During World War II, Lipscomb also worked on war-related projects. He worked with nitroglycerin propellants.
Scientific discoveries
Lipscomb worked in three main areas. These were nuclear magnetic resonance, boron chemistry and chemical bonding, and large biochemical molecules. He often took on very challenging projects.
Nuclear magnetic resonance
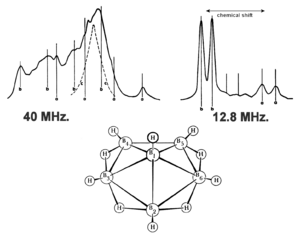
Lipscomb believed that nuclear magnetic resonance (NMR) could help discover the structures of new boron compounds. This was a faster way than using X-ray diffraction. He was partly right, but X-ray diffraction is still needed for many structures. The picture shows an NMR spectrum of a boron molecule.
Lipscomb also studied compounds called carboranes. He used NMR to see how other molecules would attach to them. This led him to develop a theory about "chemical shifts." These calculations helped find accurate values for how molecules behave in magnetic fields.
Much of this work is in a book called NMR Studies of Boron Hydrides and Related Compounds. Lipscomb wrote this book with Gareth Eaton.
Boron chemistry and chemical bonding
Lipscomb wanted to understand boron compounds better. He hoped this would help him understand other complex compounds called "intermetallics." These are compounds made of different metals. There are many of them, but scientists don't fully understand how their atoms bond. This part of his research was very difficult. However, he made big progress in understanding boron bonding. This work helped him win the Nobel Prize.
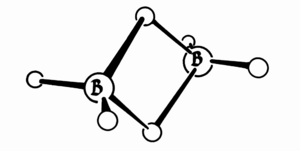
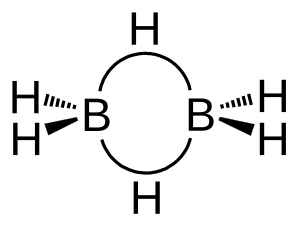
One important idea in boron chemistry is the "three-center two-electron bond." In a normal bond, two electrons connect two atoms. But in a three-center two-electron bond, two electrons connect three atoms. The diagrams show this in a molecule called diborane.
Lipscomb's group studied the structures of boron compounds. They used X-ray crystallography in the 1950s. They also created theories to explain how these unusual bonds work. They later used these methods for carboranes, which contain carbon, boron, and hydrogen.
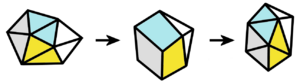
Lipscomb also solved a puzzle about "wandering atoms." Boron and hydrogen compounds often form cage-like structures. Sometimes, atoms in these cages move around a lot. Lipscomb suggested the "diamond-square-diamond" mechanism to explain this.
His group also found a new type of bond in a molecule called B10H16. This molecule had a direct bond between two boron atoms without any hydrogen atoms. This was new for boron hydrides.
Lipscomb's group developed ways to calculate how electrons behave in molecules. These calculations helped them understand boron and carborane compounds.
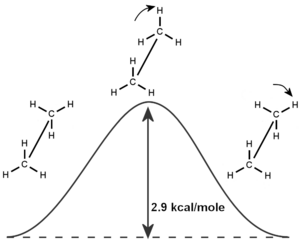
They also accurately calculated the "ethane barrier to rotation." This is how much energy it takes for parts of an ethane molecule to twist around.
Lipscomb and his team also worked on "molecular orbitals." These describe where electrons are likely to be found in a molecule. They showed how electrons can be spread out over a whole molecule.
Nobel Prize in Chemistry winner Roald Hoffmann was one of Lipscomb's students. Under Lipscomb's guidance, Hoffmann helped develop the "Extended Hückel method." This is a way to calculate molecular orbitals.
Lipscomb's work on boron hydrides and chemical bonding earned him the 1976 Nobel Prize in Chemistry. His doctoral advisor, Linus Pauling, also won a Nobel Prize in 1954 for his work on the nature of the chemical bond.
Large biological molecules
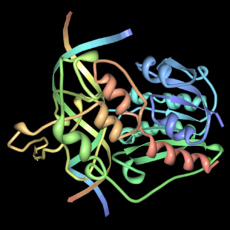
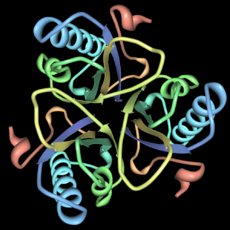
Later in his career, Lipscomb focused on the structures of proteins. He especially studied how enzymes work. Enzymes are proteins that speed up chemical reactions in living things. His group used X-ray diffraction to figure out the 3D shapes of these proteins. This helped them understand how the molecules do their jobs.
The images below show some of the protein structures Lipscomb's group discovered.
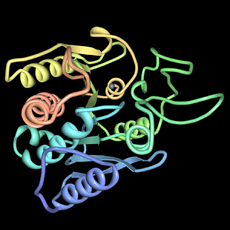
Carboxypeptidase A (left) was the first protein structure his group solved. It's a digestive enzyme made in the pancreas. It helps break down other proteins by cutting off amino acids. This was a very large molecule for its time.
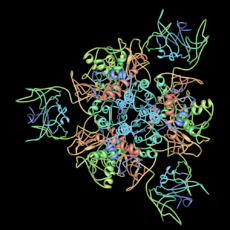
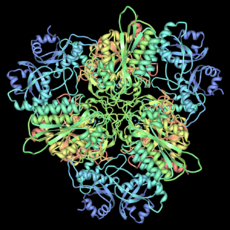
Aspartate carbamoyltransferase (right) was the second protein structure from his group. This enzyme helps build parts of DNA. It also makes sure the right amount of these parts are available. This enzyme is made of twelve smaller molecules. Six do the work, and six control how fast the work happens. This was also a very large and complex structure.
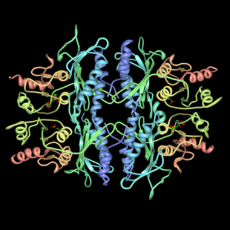
Leucine aminopeptidase (left) is similar to carboxypeptidase A. It also cuts off amino acids from proteins or peptides.
HaeIII methyltransferase (right) attaches to DNA. It adds a "methyl group" to the DNA.
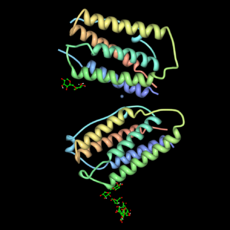
Human interferon beta (left) is released by white blood cells. It helps trigger the immune system when the body fights off germs.
Chorismate mutase (right) speeds up the making of important amino acids. These are phenylalanine and tyrosine.
Fructose-1,6-bisphosphatase (left) was studied by Lipscomb's group. They worked with a company to find a treatment for type 2 diabetes. They studied a molecule that could slow down sugar production by this enzyme.
Lipscomb's group also helped understand other important biological molecules. These include concanavalin A, glucagon, and carbonic anhydrase.
Another Nobel Prize in Chemistry winner, Thomas A. Steitz, was a student in Lipscomb's lab. Steitz helped determine the structures of carboxypeptidase A and aspartate carbamoyltransferase. Steitz later won the Nobel Prize in 2009 for his work on even larger structures, like the ribosome.
Ada Yonath, who shared the 2009 Nobel Prize with Steitz, also spent time in Lipscomb's lab. Both Steitz and Yonath were inspired there to study very large biological structures.
Other contributions
The mineral lipscombite (picture at right) was named after Professor Lipscomb. A mineralogist named John Gruner first made it artificially.
Lipscomb's lab was also a pioneer in "low-temperature X-ray diffraction." This means keeping crystals very cold while studying them with X-rays. Keeping crystals cold helps get clearer 3D images. It also protects the crystals from damage by the X-rays. This method is now a normal procedure in crystallography.
Lipscomb and his students studied many other important chemical compounds. These include hydrazine, nitric oxide, and leurocristine (Vincristine). Leurocristine is used in some cancer treatments.
Awards and honors
- Guggenheim Fellow, 1954
- Fellow of the American Academy of Arts and Sciences in 1960.
- Member of United States National Academy of Sciences
- Foreign Member of the Royal Netherlands Academy of Arts and Sciences (1976)
- Nobel Prize in Chemistry (1976)
Several books and scientific meetings have been dedicated to Lipscomb's work.
See also
 In Spanish: William Lipscomb para niños
In Spanish: William Lipscomb para niños
 | Charles R. Drew |
 | Benjamin Banneker |
 | Jane C. Wright |
 | Roger Arliner Young |