Organometallic chemistry facts for kids
Organometallic chemistry is a special part of chemistry. It looks at chemical compounds that have a direct connection, or chemical bond, between a carbon atom and a metal atom. This field mixes ideas from two other areas: inorganic chemistry (which studies compounds without carbon-hydrogen bonds) and organic chemistry (which focuses on carbon-based compounds).
Some examples of these compounds include tetraethyllead, which was once used in gasoline. Another important one is Methylcobalamin, also known as Vitamin B12.
Contents
What are Organometallic Compounds?
Organometallic compounds are special chemical compounds. They have strong chemical bonds between one or more metal atoms and one or more carbon atoms. These carbon atoms are usually part of an organic group.
You can often spot these compounds by the word "organo-" in their names. For example, there are organopalladium compounds. Some important biological compounds, like haemoglobin, are also considered organometallic.
Sometimes, you might hear about "metalorganics". These are metal compounds that don't have a direct metal-carbon bond. Instead, they have organic parts that connect to the metal in a different way.
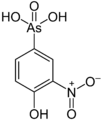
Besides traditional metals, some other elements can also form organometallic compounds. These include boron, silicon, arsenic, and selenium.
Metals and Organic Parts
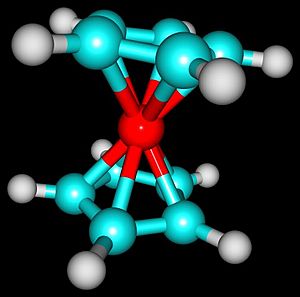
Many chemical complexes have metals connected to organic parts. Often, the organic part connects to the metal using atoms like oxygen or nitrogen. When this happens, they are called "coordination compounds."
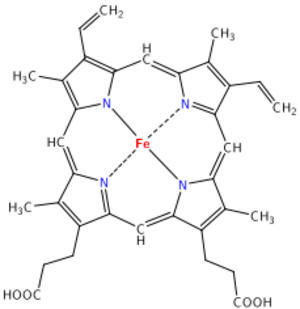
Many organic coordination compounds are found in nature. For example, hemoglobin in your blood has an iron atom connected to nitrogen atoms. Chlorophyll, which makes plants green, has a magnesium atom at its center. This area of study is called bioinorganic chemistry.
However, methylcobalamin (a type of Vitamin B12) is different. It has a direct bond between cobalt and a methyl group. This makes it a true organometallic compound, which is rare in biology.
How They Work
The bond between a metal and carbon in organometallic compounds is special. It's not fully ionic (where atoms give or take electrons) and not fully covalent (where atoms share electrons). It's somewhere in between.
This "in-between" nature makes them very useful in industry. They are stable enough to be used in solutions but also reactive enough to cause important chemical changes. Two important types are organolithium and Grignard reagents.
What Are They Used For?
Organometallic compounds are used in many practical ways. They help create new materials and make chemical reactions happen faster.
For example, all the world's polyethylene and polypropylene plastics are made using organometallic catalysts. These catalysts are like chemical helpers that speed up reactions without being used up themselves.
They also help make acetic acid, which is used in many products. And they are used to produce many synthetic alcohols.
Organometallic compounds can be very basic and good at reducing other chemicals. They are excellent at helping polymerization reactions, which create long chains of molecules.
Sometimes, organometallic compounds can be found in the environment. Scientists worry about compounds like organo-lead and organo-mercury because they can be toxic and harmful.
Scientists are always researching new ways to use organometallic catalysts. With concerns about energy and the environment, there's a lot of interest in finding "green" technologies. Many organometallic catalysts show promise in making chemical production less wasteful and creating less toxic waste.
A Look Back in Time
Scientists have been studying organometallic chemistry for a long time.
- In the 1700s, Louis Claude Cadet worked with methyl arsenic compounds.
- In the 1800s, William Christopher Zeise made a platinum-ethylene complex. Edward Frankland discovered dimethyl zinc, and Ludwig Mond found Ni(CO)4.
- Victor Grignard worked with organomagnesium compounds, which led to the important Grignard reaction.
Years ago, tetraethyllead was added to gasoline to stop engine knocking. But because lead is toxic, it's not used in gasoline anymore. Now, other organometallic compounds like ferrocene are used instead.
In 1973, Ernst Fischer and Geoffrey Wilkinson won the Nobel Prize for their work on metallocenes. This made organometallic chemistry much more popular. Later, in 2005, Yves Chauvin, Robert H. Grubbs, and Richard R. Schrock shared the Nobel Prize for their work on metal-catalyzed olefin metathesis.
Important Moments in Organometallic Chemistry
Here are some key dates in the history of organometallic chemistry:
- 1760: Louis Claude Cadet finds Cacodyl from a cobalt mineral.
- 1827: Zeise's salt, the first platinum/olefin complex, is made.
- 1848: Edward Frankland discovers diethylzinc.
- 1863: Charles Friedel and James Crafts prepare organochlorosilanes.
- 1890: Ludwig Mond discovers Nickel carbonyl.
- 1899: The Grignard reaction is introduced.
- 1900: Paul Sabatier works on using metal catalysts for hydrogenation. This helps the food industry, for example, in making margarine.
- 1912: Nobel Prize awarded to Victor Grignard and Paul Sabatier.
- 1930: Henry Gilman works on lithium cuprates, leading to the Gilman reagent.
- 1951: Ferrocene is discovered.
- 1963: Nobel Prize for Karl Ziegler and Giulio Natta for their Ziegler-Natta catalyst.
- 1965: Discovery of cyclobutadieneiron tricarbonyl.
- 1968: The Heck reaction is developed.
- 1973: Nobel Prize for Geoffrey Wilkinson and Ernst Otto Fischer for their work on sandwich compounds.
- 1981: Nobel Prize for Roald Hoffmann and Kenichi Fukui for the Isolobal Principle.
- 2005: Nobel Prize for Yves Chauvin, Robert Grubbs, and Richard R. Schrock for metal-catalyzed alkene metathesis.
- 2010: Nobel Prize for Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki for their work on palladium-catalyzed coupling reactions.
Related pages
Images for kids
See also
 In Spanish: Química organometálica para niños
In Spanish: Química organometálica para niños