History of subatomic physics facts for kids
Have you ever wondered what everything around you is made of? From the air you breathe to the stars in the sky, everything is built from tiny, tiny pieces called particles. For a long time, people thought these particles were unbreakable. But scientists have discovered that even the smallest particles can change or break apart, creating new ones!
Scientists keep finding smaller and smaller particles. We know that everything is made of molecules. Molecules are made of atoms. And atoms are made of even smaller pieces called subatomic particles, like the atomic nuclei and electrons. But wait, there's more! Many subatomic particles (except electrons) are actually made of even tinier bits called quarks.
The study of these super-tiny particles is called particle physics. When scientists focus on the center of atoms, called the nucleus, and its parts like protons and neutrons, that's nuclear physics.
Contents
Early Ideas About Tiny Particles
The idea that everything is made of tiny, basic particles is very old! People in ancient India, like the Jains, thought about this thousands of years ago. They believed that non-living things were made of invisible particles called permanu. These permanu had color, smell, taste, and texture, and they could join together to form bigger things.
Ancient Greek thinkers like Democritus also had similar ideas about "atoms," meaning "uncuttable." Later, scientists in the Islamic world and early modern Europe, like Isaac Newton, explored these ideas too. Some even thought light was made of particles!
However, these early ideas were mostly based on thinking and philosophy, not on experiments.
In the 1800s, a scientist named John Dalton did experiments with chemicals. He figured out that each chemical element (like oxygen or iron) was made of its own special type of particle. He called these particles "atoms," just like the ancient Greeks. Dalton thought these atoms were the smallest, most basic parts of nature.
But near the end of the 1800s, scientists made an amazing discovery: Dalton's atoms were not the smallest! They were actually made of even tinier pieces.
From Atoms to Nucleons
Understanding Electricity and Magnetism
Throughout the 1800s, scientists learned a lot about electricity and magnetism. James Clerk Maxwell put all these ideas together into a complete theory. This theory described electricity and magnetism as continuous fields, like invisible waves. Later, Hendrik Antoon Lorentz created a theory based on tiny charged particles called "ions," which helped explain Maxwell's ideas.
The Electron: A Tiny Discovery
The electron was discovered between 1879 and 1897 by scientists like J. J. Thomson. It was found to be a tiny particle with a negative electric charge. In 1909, Robert Andrews Millikan and Harvey Fletcher carefully measured its charge using their famous oil drop experiment.
Scientists soon realized that these negatively charged electrons were part of atoms, along with some unknown positively charged material. This was a big step! The electron became the first truly fundamental particle ever found.
Radioactivity: Atoms That Change
Around the same time, scientists discovered "radioactivity." This showed that some elements could naturally change into other elements through a process called radioactive decay. This was another clue that atoms weren't unbreakable.
Even with these discoveries, the name "atom" stuck. Today, an atom means the smallest particle of a chemical element, even though we know it can be broken down further.
Looking Inside the Atom
In the early 1900s, scientists only knew about two main forces: electromagnetism (which explains how magnets and electricity work) and gravitation (which explains why things fall). Gravity couldn't explain how atoms held together. So, scientists thought the positive parts of an atom must attract the negative electrons using the electromagnetic force.
In 1909, Ernest Rutherford and Thomas Royds showed that alpha particles (which are like tiny helium atoms without their electrons) could combine with electrons to form full helium atoms.
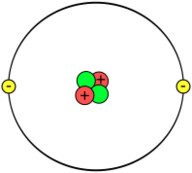
A very important experiment in 1907, called the gold foil experiment by Rutherford, showed something surprising. Atoms are mostly empty space! Almost all of an atom's mass is packed into a tiny, dense center called the atomic nucleus.

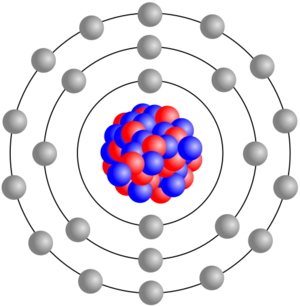
By 1914, experiments by Rutherford and others confirmed that an atom has a positively charged nucleus surrounded by much lighter electrons. These discoveries helped explain radioactive decay and how elements can change. It turned out that the atomic number of an element (like 6 for carbon or 8 for oxygen) is simply the positive charge of its nucleus.
Chemical changes don't affect the nucleus, which is why elements stay the same chemically. But if the nucleus changes its charge or mass (by giving off or taking in a particle), the atom can become a different element! Special relativity helped explain how the "missing mass" in these changes turns into energy.
The study of how nuclei change and what they're made of is called nuclear physics. The study of the whole atom, including its electrons, is called atomic physics. New ideas in quantum physics, like the Bohr model, helped us understand how electrons are arranged in atoms, which explains all of chemistry.
In 1918, Rutherford confirmed that the nucleus of a hydrogen atom was a single particle with a positive charge. He named it the proton. Scientists also found that elements could have different versions called isotopes. Isotopes of the same element have the same number of protons but different masses. This led Rutherford to guess that nuclei (other than hydrogen) must also contain particles with no charge. He called these the neutrons.
Evidence grew that atomic nuclei are made of these smaller particles, now called nucleons (protons and neutrons). It became clear that while protons push each other away (because they both have positive charges), nucleons attract each other with a new, very strong force called the nuclear force.
These discoveries led to major breakthroughs, like nuclear fission (splitting atoms) in 1939 and nuclear fusion (joining atoms) in the same year. These discoveries opened the door to making new elements and also led to the development of nuclear weapons.
Quantum Mechanics and New Discoveries

1s 2s 2p (3 items).
All complete subshells (including 2p) are inherently spherically symmetric, but it is convenient to assign to "distinct" p-electrons these two-lobed shapes.
To truly understand atoms and nuclei, scientists needed to learn more about what particles really are. Experiments and new theories, like Erwin Schrödinger's idea of "electron waves," showed that there's no real difference between particles and waves. This is called wave–particle duality. For example, light, which we think of as a wave, can also be described as particles called photons.
Another huge discovery was that particles of the same type are truly identical particles. All electrons are exactly alike. You can't tell one electron from another! This is also true for protons and neutrons. This suggested that there's only a limited number of basic particle types in the universe.
Scientists also found that particles can be divided into two main groups based on a property called spin:
- Bosons: These particles often carry forces, like the photon that carries light.
- Fermions: These particles make up matter, like electrons, protons, and neutrons.
Spin is like an internal rotation of a particle, even if it's not actually spinning. It's a fundamental property that helps tell particles apart.
With this new understanding, physicists made bold predictions. Paul Dirac predicted the existence of the positron (an "anti-electron") in 1928, and Wolfgang Pauli predicted the neutrino in 1930. Both were later found, proving these theories right!
These ideas led to a powerful framework called quantum field theory. The first complete theory of this kind, quantum electrodynamics, perfectly explained how atoms work, including the Periodic Table and how atoms give off light. These quantum ideas were also applied to nuclear physics, explaining things like alpha decay and the strong nuclear force.
From Nuclei to Nuclear Engineering
Scientists developed models to predict the properties of atomic nuclei. While it's still hard to calculate everything perfectly, these models helped us understand how nuclei behave.
In the 1940s, the study of nuclear fission (splitting atoms) became very important, especially for military uses. This led to the development of nuclear fission reactors and fission bombs. This field grew into what we now call nuclear engineering. Scientists also created new, heavier elements called transuranium elements during this time.
Making even heavier elements (beyond fermium) requires powerful particle accelerators to smash atomic nuclei together. This research continues today, but mainly for scientific purposes.
Another important area of nuclear physics is nuclear fusion (joining atoms). This is related to thermonuclear weapons and the hope of creating clean thermonuclear energy. It also helps us understand how stars produce energy and how elements were formed in the early universe.
Physics Goes to High Energies
Strange Particles and Weak Forces
In the 1950s, with better particle accelerators and studies of cosmic rays, scientists could smash particles together at very high energies. These collisions created many new, short-lived particles. Some of these, like hyperons and K-mesons, had unusually long lifetimes. Scientists found this was due to a new property they called strangeness. Strangeness is conserved in most interactions but not in the weak interaction. These "strange" particles and the muon were the first hints of what we now call the second generation of fundamental particles.
The weak interaction also revealed another puzzle: it doesn't always follow mirror symmetry. This means that if you look at a weak interaction in a mirror, it might not look like a possible real interaction!
Throughout the 1950s and 1960s, so many new particles were discovered that scientists called it the "particle zoo"! It became clear that most of these particles were not truly elementary but were made of even smaller, unseen parts.
Deeper Inside Matter: Quarks
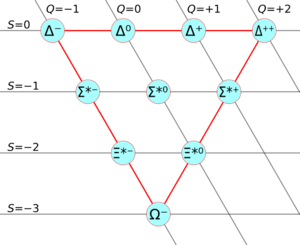
Scientists like Murray Gell-Mann started to organize these many new particles, especially the mesons and baryons. He developed a system called the "Eightfold Way" which helped predict new particles. The most famous prediction was the Error no link defined particle, which was found in 1964. This led to the idea of quarks.
The quark model suggested that protons, neutrons, and many other particles are made of quarks. A new theory called quantum chromodynamics (QCD) was developed in the 1970s. It explained the strong interaction, the force that holds quarks together, which is carried by particles called gluons.
An interesting thing about quarks is that we can't see them on their own. They are always stuck together inside other particles (this is called color confinement). This is different from atoms, where electrons and nuclei can be separated.
With the quark model, the term "elementary particle" became more specific. It now refers only to leptons (like electrons), quarks, their antiparticles, and the particles that carry fundamental forces. All the other particles in the "zoo" are now called subatomic particles, and many of them are hadrons (particles made of quarks).
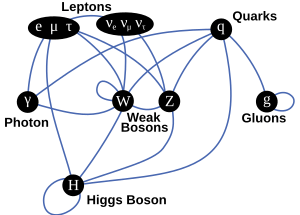
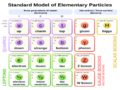
Quarks, Leptons, and Four Fundamental Forces
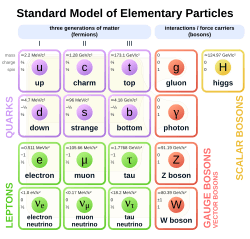
Because quantum field theory says there's no real difference between particles and forces, classifying elementary particles also helped classify the forces of nature.
Today, we understand that a huge number of particles and forces are actually combinations of a small number of basic things:
- Fundamental bosons: These carry the fundamental forces.
- Quarks: These are building blocks of matter.
- Leptons: These are also building blocks of matter, like electrons.
Scientists then figured out which elementary particles are affected by which forces:
- Gravitation: All particles are affected by gravity.
- Electromagnetic interaction: All charged elementary particles are affected by this force. Even neutrons, which have no overall charge, are affected because they are made of charged quarks.
- Weak interaction: All fermions (matter particles) are affected by this force.
- Strong interaction: Only quarks and gluons (which carry the strong force) are affected by this force.
Scientists dreamed of a "united field theory" that would combine all these forces. The first successful step was the electroweak theory, which combined the electromagnetic and weak forces. This led to the Standard Model in the 1970s, which also included the strong interaction. So, the Standard Model covers three of the four fundamental forces!
The discovery of the particles that carry the weak force (the W and Z bosons) in the 1980s confirmed the Standard Model even more.
While the Standard Model has been incredibly successful, it doesn't include gravity. Scientists are still looking for a theory that can combine gravity with the other three forces. Ideas like supersymmetry and string theory have been explored, but so far, experiments at places like the Large Hadron Collider haven't found evidence for them. This means physicists are still searching for new ideas to solve these big questions!
The Higgs Boson: Giving Particles Mass
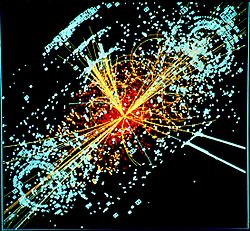
For a long time, the Higgs boson was the only particle predicted by the Standard Model that hadn't been found. Scientists believed it was the particle of a special field, the Higgs field, that gives other particles their rest masses. Without this field, all elementary particles would zoom around at the speed of light, and there would be no atoms or life!
On July 4, 2012, physicists at CERN's Large Hadron Collider announced they had found a new subatomic particle that looked a lot like the Higgs boson! It was a huge moment in physics. This particle was very heavy, weighing 125 billion electron volts.
Scientists were very excited because this discovery could help us understand why particles have mass and how our universe came to be. They called it a "Higgslike" particle at first, because they needed more experiments to be sure it was the exact Higgs boson predicted by the Standard Model. The chance of this discovery being a random fluke was incredibly small, less than one in 3.5 million!
Michael Turner, a cosmologist, said this was a "big moment for particle physics and a crossroads." It could be the biggest discovery in decades, pointing the way to even deeper ideas about reality.
The Higgs boson was predicted in 1964 by six physicists, including Peter Higgs. Their theory said that if you hit the invisible Higgs field with enough energy, it would produce its own quantum particle. This particle would be very fragile and break apart quickly. The challenge was that the theory didn't say how much the Higgs boson should weigh, making it very hard to find.
After more experiments, in March 2013, it was tentatively confirmed that the newly discovered particle was indeed the Higgs boson.
Even though we can't "see" Higgs fields, they are important in theories about the universe. They might have caused a huge burst of expansion called inflation in the early universe and could even be related to the mysterious dark energy that is speeding up the universe's expansion today.
Images for kids
-
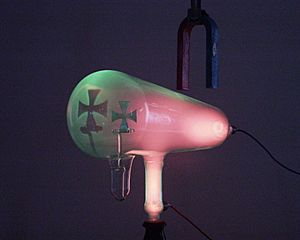
A Crookes tube with a magnetic deflector
-
Cloud chambers played an important role as particle detectors in the early days of subatomic physics. Some particles including the positron were even discovered by using this device
-
The Standard Model
-
One possible signature of a Higgs boson from a simulated proton–proton collision. It decays almost immediately into two jets of hadrons and two electrons, visible as lines.
 | Bayard Rustin |
 | Jeannette Carter |
 | Jeremiah A. Brown |