Lothar Meyer facts for kids
Quick facts for kids
Lothar Meyer
|
|
|---|---|

Meyer in 1883
|
|
| Born |
Julius Lothar Meyer
19 August 1830 Varel, Duchy of Oldenburg
|
| Died | 11 April 1895 (aged 64) |
| Nationality | German |
| Alma mater | University of Würzburg, University of Breslau |
| Known for | Periodic table of chemical elements |
| Awards | Davy Medal (1882) |
| Scientific career | |
| Fields | Chemistry |
| Institutions | University of Tübingen |
| Influences | Robert Bunsen |
Julius Lothar Meyer (born August 19, 1830 – died April 11, 1895) was an important German chemist. He was one of the first scientists to create a version of the periodic table of chemical elements. Another famous chemist, Dmitri Mendeleev, also worked on the periodic table around the same time. Both Meyer and Mendeleev had studied with Robert Bunsen. Lothar Meyer was known by his middle name, Lothar, throughout his life.
Contents
Lothar Meyer's Early Life and Studies
Lothar Meyer was born in Varel, Germany. His father, Friedrich August Meyer, was a doctor. Lothar first studied medicine at the University of Zurich in 1851. Later, he went to the University of Würzburg to study pathology, which is the study of diseases.
He then moved to the University of Heidelberg, where the famous chemist Robert Bunsen taught. In 1858, Meyer earned his Ph.D. in chemistry from the University of Breslau. His research was about how carbon monoxide affects blood. He discovered that oxygen combines with hemoglobin in the blood, which is important for breathing.
Meyer also studied mathematical physics at the University of Königsberg. In 1859, he became a university teacher (called a Privatdozent) in physics and chemistry at the University of Breslau.
Meyer's Career as a Professor
In 1866, Meyer started working at the Eberswalde Forestry Academy. Two years later, he became a professor at the Karlsruhe Polytechnic.
During the Franco-Prussian War, the Polytechnic building was used as a hospital. Meyer helped care for the wounded soldiers. In 1876, he became a Professor of Chemistry at the University of Tübingen. He worked there until he passed away in 1895 at age 64.
Developing the Periodic Table
Lothar Meyer is most famous for his work on the periodic table of elements. He noticed that if elements were put in order by their atomic weight, they showed similar chemical and physical properties that repeated regularly. This idea is called "periodicity."
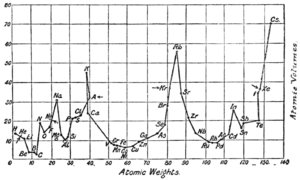
Meyer created a graph showing the atomic volumes of elements compared to their atomic weights. This graph clearly showed how the properties of elements repeated in a pattern. Elements with similar properties appeared at similar points on the curve.
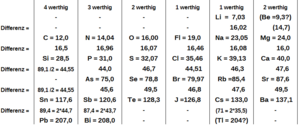
Meyer started writing his book, Die modernen Theorien der Chemie (Modern Theories of Chemistry), in 1862. It was published two years later. This book included an early version of the periodic table with 28 elements. For the first time, elements were grouped based on their valence, which describes how they combine with other elements.
He published different versions of his classification table. In 1864, he showed it in a horizontal form. In 1870, he published a vertical form.
Meyer's 1864 Periodic Table
This table shows how Meyer grouped elements by their combining power (valence).
| Valence IV | Valence III | Valence II | Valence I | Valence I | Valence II | Mass difference | |
| I row | Li | Be | ~16 | ||||
| II row | C | N | O | F | Na | Mg | ~16 |
| III row | Si | P | S | Cl | K | Ca | ~45 |
| IV row | As | Se | Br | Rb | Sr | ~45 | |
| V row | Sn | Sb | Te | I | Cs | Ba | ~90 |
| VI row | Pb | Bi | Tl | ~90 |
Meyer's 1870 Periodic Table
In 1869, Dmitri Mendeleev published his own periodic table. A few months later, Meyer published a new version of his table. This one included almost all the elements known at the time and was very similar to Mendeleev's table.
| I | II | III | IV | V | VI | VII | VIII | IX |
| B | Al | ?In |
Tl | |||||
| C | Si | Ti |
Zr |
Sn | Pb | |||
| N | P | V |
As | Nb |
Sb | Ta |
Bi | |
| O | S | Cr |
Se | Mo |
Те | W |
||
| F |
Cl |
Mn Fe Co Ni |
Br |
Ru Rh Pd |
I |
Os Ir Pt |
||
| Li | Na | K | Cu |
Rb | Ag |
Cs | Au |
|
?Be |
Mg | Ca | Zn |
Sr | Cd |
Ba | Hg |
|
 The question marks indicate that the atomic weights The question marks indicate that the atomic weightswere conjectured based on the equivalent weights. |
||||||||
Meyer developed his full periodic table on his own. However, he recognized that Mendeleev had published his table first. Meyer's paper also included a graph showing how atomic volumes changed with atomic weights. This graph clearly showed the repeating patterns of elements. Like Mendeleev, Meyer also predicted that new elements would be discovered.
In 1882, both Lothar Meyer and Dmitri Mendeleev received the Davy Medal from the Royal Society. This award honored their important work on the Periodic Law, which explains how elements are organized.
The mineral lotharmeyerite was discovered in 1983. It was named after Meyer to honor his contributions to the Periodic Law. Several similar minerals have also been named after him.
Lothar Meyer's Personal Life
Lothar Meyer married Johanna Volkmann in 1866.
Tributes to Lothar Meyer
On August 19, 2020, Google celebrated Lothar Meyer's 190th birthday with a special Google Doodle on their homepage.
See also
 In Spanish: Julius Lothar Meyer para niños
In Spanish: Julius Lothar Meyer para niños
 | Georgia Louise Harris Brown |
 | Julian Abele |
 | Norma Merrick Sklarek |
 | William Sidney Pittman |