Geochemistry facts for kids
Geochemistry is a cool science that mixes chemistry and geology to understand how our Earth and even the whole Solar System work. It helps us figure out things like how planets formed, why we have different rocks like granite and basalt, and how the Earth's mantle (the layer beneath the crust) moves. It's all about studying the chemical makeup of rocks, water, and even the air!
The Story of Geochemistry

The word "geochemistry" was first used way back in 1838 by a scientist named Christian Friedrich Schönbein. But for a long time, people just called it "chemical geology." Geologists and chemists didn't really work together much at first.
Geochemistry really started to grow when big labs were set up. One important one was the United States Geological Survey (USGS) in 1884. They began carefully studying the chemistry of rocks and minerals. Frank Wigglesworth Clarke, a top chemist at the USGS, noticed that elements with heavier atomic weights were usually less common. He wrote a famous book called The Data of Geochemistry.
Scientists also started looking at meteorites (rocks from space) around 1850. They compared them to Earth rocks. In 1901, Oliver C. Farrington thought that even though meteorites and Earth rocks were different, the *relative* amounts of elements in them should be similar. This idea helped start a new field called cosmochemistry, which teaches us a lot about how Earth and the Solar System were made.
In the early 1900s, scientists like Max von Laue and William L. Bragg discovered how to use X-rays to see the structure of crystals. Then, in the 1920s and 1930s, Victor Goldschmidt and his team used these methods to study many common minerals. They created rules about how elements group together. Goldschmidt published his findings in a series of books.
Later, from the 1960s to 2002, Manfred Schidlowski studied the chemistry of the Early Earth and how life affected it. He looked at isotopes to find clues about the first living things from the Precambrian era.
Different Types of Geochemistry
Geochemistry has many specialized areas, like different branches of a tree:
- Aqueous geochemistry studies how elements like copper, sulfur, and mercury move through water systems like rivers and oceans. It looks at how they interact with the air and land.
- Biogeochemistry focuses on how living things (like plants and animals) affect the Earth's chemistry.
- Cosmochemistry explores how elements and their different forms (isotopes) are spread out across the whole cosmos.
- Isotope geochemistry measures the amounts of different elements and their isotopes (atoms of the same element with different numbers of neutrons) in Earth's rocks and surface.
- Organic geochemistry studies the role of chemicals that come from living or once-living organisms.
- Photogeochemistry looks at chemical reactions on Earth's surface that are triggered by light.
- Regional geochemistry applies geochemical ideas to study the environment, water systems, and to find new mineral deposits.
Understanding Chemical Elements
The basic building blocks of everything are chemical elements. Each element is unique because of its atomic number (Z), which is the number of protons in its center, called the nucleus.
An element can have different numbers of neutrons in its nucleus. Atoms of the same element with different neutron numbers are called isotopes. For example, two common isotopes of chlorine are 35Cl and 37Cl. In geochemistry, stable isotopes help scientists track how chemicals move and react. Radioactive isotopes, which are unstable, are mainly used to figure out the age of samples.
The way an atom behaves chemically – how it connects with other elements and what kind of bonds it forms – depends on its electrons. These arrangements are shown in the periodic table. Elements are grouped into categories like alkali metals, transition metals, and noble gases based on their electron setup.
Another helpful way to group elements in geochemistry is the Goldschmidt classification. It puts elements into four main groups:
- Lithophiles love to combine with oxygen. These elements, like Na, K, and Si, are common in the Earth's crust. They form minerals like silicates.
- Siderophile elements (Fe, Co, Ni) like to mix with iron. They tend to gather in the core.
- Chalcophile elements (Cu, Ag, Zn) form sulfides.
- Atmophile elements (O, N, H, and noble gases) are found mostly in the atmosphere.
Within these groups, some elements are refractory, meaning they stay stable even at very high temperatures. Others are volatile, meaning they evaporate easily. This difference allows them to be separated by heat.
Earth's Changing Chemistry: Differentiation and Mixing
The chemical makeup of Earth and other planets is shaped by two opposite processes: differentiation (separating) and mixing.
In Earth's mantle, differentiation happens at places like mid-ocean ridges. Here, some parts of the mantle melt, and the materials that are harder to melt stay behind. The melted part rises to form new rock like basalt. When an oceanic plate sinks back into the mantle, convection currents eventually mix these different parts together again.
On the surface, Erosion separates rocks. For example, granite can break down into clay (which ends up on the ocean floor), sandstone (on continent edges), and dissolved minerals in ocean water. But then, processes like Metamorphism (changing rocks with heat and pressure) can mix these elements back together. In the ocean, living things can cause chemical separation, but when they dissolve, the materials mix again.
Fractionation: Uneven Distribution
A big reason for chemical differences is fractionation. This is when elements or isotopes are not spread out evenly. It can happen because of chemical reactions, changes in physical state (like melting), or even radioactivity.
On a huge scale, planetary differentiation is when a planet separates into different chemical layers. For example, Earth and other rocky planets formed an iron-rich core and a mantle and crust rich in silicates. In Earth's mantle, the main way chemicals separate is through partial melting, especially near mid-ocean ridges. This happens when only part of a solid rock melts, and the liquid part separates from the solid.
Isotopic fractionation is when different isotopes of an element separate. Atoms with heavier isotopes are more stable. So, in chemical reactions, heavier isotopes prefer to be in compounds with a higher oxidation state. In phase changes, heavier isotopes tend to gather in the heavier phases. This effect is biggest for light elements because the mass difference between their isotopes is a larger part of their total mass.
Scientists often compare isotope ratios to a standard. For example, sulfur has two common stable isotopes: 32S and 34S. The ratio of these isotopes in a sample is compared to a standard ratio, and the difference is reported in "parts per mil" (‰), which is like parts per thousand.
Equilibrium Fractionation
Equilibrium fractionation happens when chemicals or different states of matter (like liquid and vapor) are in balance with each other. In this situation, heavier isotopes tend to gather in the heavier phase. For example, when water evaporates, the liquid water will have slightly more of the heavier oxygen (18O) and hydrogen (2H) isotopes than the water vapor. This separation is usually stronger at lower temperatures.
Kinetic Fractionation
Kinetic fractionation happens when things are not in balance, like during a fast chemical reaction. Lighter isotopes usually react faster because their bonds are a bit weaker. This means the reaction products will have more of the lighter isotopes.
A special type of kinetic fractionation is biological fractionation. Living organisms often prefer lighter isotopes because it takes less energy to break their chemical bonds. The environment and the type of organism can also greatly affect this process.
Geochemical Cycles: Earth's Recycling System
Chemical elements are constantly moving and changing in what we call geochemical cycles. These cycles involve many physical and chemical processes. To understand them, scientists use both observations and theoretical models.
Instead of tracking every single atom, geochemists divide the Earth into "geochemical reservoirs" – like big storage tanks for elements. For example, the ocean can be one reservoir, or it can be split into several smaller ones. In a "box model," each reservoir is like a box with materials flowing in and out.
Geochemical models often involve feedback. Imagine a simple cycle where salt is removed from the ocean when it forms evaporites (salt deposits). The faster salt is removed, the more salt there is in the ocean. If the input of salt is constant and the output depends on the amount of salt in the ocean, the system will eventually reach a steady state where the amount of salt stays roughly the same.
The residence time is how long, on average, an element stays in a reservoir. For example, molybdenum stays in the ocean for about 800,000 years, while aluminum stays for only 100 to 1,000 years because it interacts strongly with particles.
How Elements Are Spread Out
In Our Solar System
The makeup of our Solar System is similar to many other stars. We believe it formed from a giant cloud of gas and dust called a solar nebula that had a uniform composition. The Sun's outer layer, called the photosphere, has a similar composition to the rest of the Solar System.
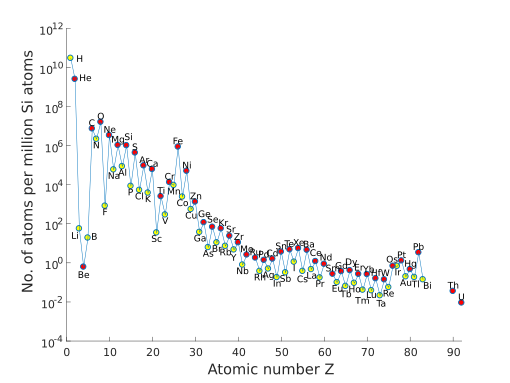
By far, the most common elements in the Solar System are hydrogen (about 75%) and helium (about 24%). All other elements make up only about 1.3%! Generally, elements become less common as their atomic number (number of protons) increases. Also, elements with an even atomic number are more common than their odd-numbered neighbors.
Most of the hydrogen, helium, and some lithium were made right after the Big Bang. All the other elements were created inside stars through processes like nuclear fusion.
What Meteorites Tell Us
Meteorites come in many different types. Scientists can analyze them to see if they came from planetesimals (small early planets) that melted or separated into layers.
Chondrites are a special type of meteorite that haven't changed much since the early Solar System. They are about 4.56 billion years old. One type, called a CI chondrite, has a composition that almost perfectly matches the Sun's photosphere, except for some elements that easily evaporate (like hydrogen and helium) and a few others that are destroyed in the Sun. Because of this, CI chondrites are considered the best example of what the early Solar System was made of.
The Giant Planets
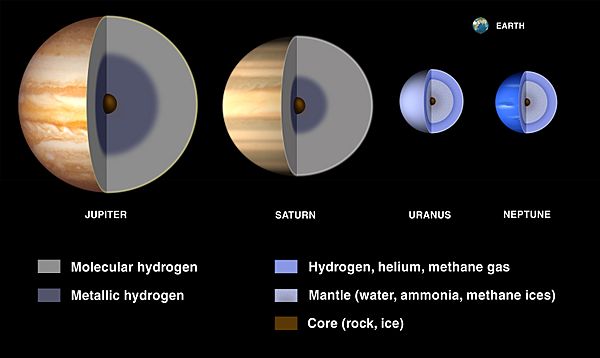
The planets in our Solar System are split into two groups. The four inner planets (Mercury, Venus, Earth, and Mars) are small and rocky. The four outer planets are the giant planets, which are mostly made of hydrogen and helium and are much less dense. These giant planets are further divided into gas giants (Jupiter and Saturn) and ice giants (Uranus and Neptune), which have large icy cores.
Most of what we know about the giant planets' composition comes from looking at their light using spectroscopy. Since the 1930s, we've known Jupiter has hydrogen, methane, and ammonium. Later, better tools allowed us to find many more molecules, like ethane and water. However, it's harder to study the more distant planets because the sunlight they reflect is very dim.
Helium is hard to detect from Earth, so we only found out about its abundance on giant planets when spacecraft like Galileo (to Jupiter in 1995) and Cassini (to Saturn in 2017) were sent there. The Galileo probe found that Jupiter's atmosphere had less helium and neon than expected, but more carbon, nitrogen, and sulfur.
Spectroscopy only lets us see the very top layers of Jupiter and Saturn's atmospheres. To understand what's inside, scientists create models using temperature data and equations that describe how materials behave under extreme pressure. These models suggest that hydrogen becomes a metallic liquid deep inside Jupiter and Saturn. In Uranus and Neptune, it stays in a molecular state.
Current models suggest that all four giant planets have rocky and icy cores that are roughly the same size. However, the amount of hydrogen and helium decreases from Jupiter (about 300 times Earth's mass) to Saturn (75 times Earth's mass) and then to Uranus and Neptune (only a few times Earth's mass). This means that while gas giants are mostly hydrogen and helium, ice giants are mostly made of heavier elements like oxygen, carbon, nitrogen, and sulfur, often in the form of water, methane, and ammonia.
The Rocky Planets
The rocky planets (Mercury, Venus, Earth, and Mars) are thought to have formed from the same material as the giant planets, but they lost most of their lighter elements and have different histories. Planets closer to the Sun might have more elements that are stable at high temperatures.
We get information about Mars, Venus, and Mercury from spacecraft missions. For example, the Mars Odyssey orbiter measured the composition of Mars's crust. We also learn about Mars from meteorites that have landed on Earth. The total mass of the planets and how their elements are spread out inside also help scientists figure out their composition.
The planets formed as the solar nebula cooled. Materials condensed (turned from gas to solid) in different stages. The first materials to condense were rich in elements like calcium and aluminum. Then came nickel and iron, followed by magnesium silicates. Below certain temperatures, other compounds like FeS and volatile-rich metals formed. The compositions of the planets and the Moon are "chondritic," meaning that the ratios of elements within these groups are similar to those found in carbonaceous chondrites (a type of meteorite).
Scientists use different models to estimate planetary compositions. One idea, the "equilibrium condensation" model, suggests each planet formed in a "feeding zone" where the temperature determined which solids condensed. For example, Mercury formed at very high temperatures, so it has a lot of pure metallic iron. However, this model has a problem: it suggests planets wouldn't have atmospheres, which we know isn't true for Earth.
Another idea is "chondritic mixing" models, which use the compositions of chondrites to estimate planetary compositions. These models try to mix different types of chondrites to match what we see in the planets.
Earth's Crust: What It's Made Of
The Earth's crust is mostly made of oxides (compounds with oxygen). Things like chlorides and sulfides are rare. By 1911, Frank Wigglesworth Clarke calculated that over 47% of the Earth's crust is oxygen. It's usually combined with other elements. The main oxides are silica, alumina, iron oxides, and various carbonates (like calcium carbonate).
Silica acts like an acid, forming silicates, which are the most common minerals in igneous rocks (rocks formed from cooled magma). Based on many rock analyses, Clarke estimated the average composition of Earth's crust:
- SiO2 (silica): 59.71%
- Al2O3 (alumina): 15.41%
- Fe2O3 (iron oxide): 2.63%
- FeO (iron oxide): 3.52%
- MgO (magnesium oxide): 4.36%
- CaO (calcium oxide): 4.90%
- Na2O (sodium oxide): 3.55%
- K2O (potassium oxide): 2.80%
- H2O (water): 1.52%
- TiO2 (titanium dioxide): 0.60%
- P2O5 (phosphorus pentoxide): 0.22%
These oxides combine in many ways. For example, potash (potassium carbonate) and soda (sodium carbonate) often form feldspars. If there's extra silica, it will form quartz. If there's extra alumina, it forms corundum. These are general rules, but there are many exceptions.
Minerals in the Crust
Most of Earth's crust (90%) is made of silicate minerals. Here are their approximate abundances:
- plagioclase feldspar: 39%
- alkali feldspar: 12%
- quartz: 12%
- pyroxene: 11%
- amphiboles: 5%
- micas: 5%
- clay minerals: 5%
The remaining 3% of silicate minerals are other types. Only 8% of the Earth's crust is made of non-silicate minerals like carbonates, oxides, and sulfides.
The conditions when rocks form also play a role. Some minerals are only found deep inside the Earth. Also, the same chemical composition can lead to completely different minerals depending on the pressure and temperature.
Types of Igneous Rocks
Igneous rocks are classified based on their silica content:
- Felsic rocks contain the most silica (over 66%) and often have free quartz. Granite is an example.
- Mafic rocks have the least silica (20% or less) and a lot of magnesium and iron. They usually don't have quartz but often contain olivine. Basalt is a common mafic rock.
- Intermediate rocks are in between, generally without much quartz or olivine.
There are also "alkali" rocks, which have a lot of alkalis (like sodium) and special minerals like nepheline and leucite. "Ultramafic" rocks are very rich in olivine and have very little silica.
Most rocks, except ultramafic ones, contain feldspars or similar minerals. In felsic rocks, common feldspars are orthoclase and oligoclase, which are rich in silica and alkalis. In mafic rocks, labradorite and anorthite are common, which are rich in lime and poor in silica.
Here's a simplified table of common igneous rocks:
| Most Common Minerals | Felsic | Intermediate | Mafic | Ultramafic | |
|---|---|---|---|---|---|
| Quartz Orthoclase (and Oligoclase), Mica, Hornblende, Augite |
Little or no Quartz: Orthoclase hornblende, Augite, Biotite |
Little or no Quartz: Plagioclase Hornblende, Augite, Biotite |
No Quartz Plagioclase Augite, Olivine |
No Felspar Augite, Hornblende, Olivine |
|
| Plutonic or Abyssal type | Granite | Syenite | Diorite | Gabbro | Peridotite |
| Intrusive or Hypabyssal type | Quartz-porphyry | Orthoclase-porphyry | Porphyrite | Dolerite | Picrite |
| Lavas or Effusive type | Rhyolite, Obsidian | Trachyte | Andesite | Basalt | Komatiite |
Rocks with leucite or nepheline (which replace feldspar) are not in this table. They are usually intermediate or mafic.
Here's a table for rocks containing Nepheline and Leucite:
| Most Common Minerals | Alkali Feldspar, Nepheline or Leucite, Augite, Hornblend, Biotite | Soda Lime Feldspar, Nepheline or Leucite, Augite, Hornblende (Olivine) | Nepheline or Leucite, Augite, Hornblende, Olivine |
|---|---|---|---|
| Plutonic type | Nepheline-syenite, Leucite-syenite, Nepheline-porphyry | Essexite and Theralite | Ijolite and Missourite |
| Effusive type or Lavas | Phonolite, Leucitophyre | Tephrite and Basanite | Nepheline-basalt, Leucite-basalt |
This way of classifying rocks is based on the minerals they contain. It's a bit artificial, but it's how the science developed and is still used today. Different rock types often blend into each other, so there are many "transition" rocks with special names.
Tiny Metals in the Ocean
Trace metals are elements found in very small amounts. In the ocean, these metals easily form complexes (groups of atoms bonded together) with other common ions like hydroxide and carbonate. How these complexes form changes depending on whether the water is oxidized (has oxygen) or reduced (lacks oxygen).
When metals form strong complexes, especially with special molecules called chelates, they become less reactive. This helps keep the metals dissolved in the water instead of settling into solids.
The amounts of trace metals like cadmium, copper, molybdenum, and uranium in ocean sediments can tell us about the ocean's past oxygen levels. For example, high levels of cadmium in marine sediments might mean the ocean had low oxygen in the past.
In the ocean, dissolved trace metals can have three main types of distributions:
- Conservative-type: These metals have high concentrations and are not used much by living things. Molybdenum is an example. It stays in the ocean for about 800,000 years and is spread almost evenly throughout the water.
- Nutrient-type: These metals are strongly linked to how organic matter cycles, especially how plankton use them. Their lowest concentrations are at the ocean surface, where plankton absorb them. As dead plankton sink and break down, these metals increase in concentration deeper in the ocean. Zinc is an example, with residence times of thousands to hundreds of thousands of years.
- Scavenged-type: These metals interact strongly with particles and don't stay in the ocean for long. Aluminium is an example, with residence times of only 100 to 1,000 years. Their concentrations are highest near the seafloor, hydrothermal vents, and rivers. Dust from the atmosphere is a major source of aluminum in the ocean.
Iron and copper show a mix of these distributions. They are recycled but also strongly "scavenged" (removed by particles). Iron is a key nutrient that limits growth in many ocean areas. It's found in high amounts near hydrothermal vents, often forming iron sulfides.
Using special techniques, scientists can see that important trace metals like zinc, cobalt, cadmium, iron, and copper are often bound by organic molecules in surface seawater. These organic complexes reduce how much of the metal is available for living things to use. For example, copper can be toxic to tiny ocean plants (phytoplankton), but forming organic complexes reduces the amount of harmful copper.
See also
 In Spanish: Geoquímica para niños
In Spanish: Geoquímica para niños