Fluorine facts for kids

Liquid fluorine (at extremely low temperatures)
|
|||||||||||||||||||||
| Fluorine | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | |||||||||||||||||||||
| Allotropes | alpha, beta (see Allotropes of fluorine) | ||||||||||||||||||||
| Appearance | gas: very pale yellow liquid: bright yellow solid: transparent (beta), opaque (alpha) |
||||||||||||||||||||
| Standard atomic weight Ar, std(F) | 18.998403163(6) | ||||||||||||||||||||
| Fluorine in the periodic table | |||||||||||||||||||||
|
|||||||||||||||||||||
| Atomic number (Z) | 9 | ||||||||||||||||||||
| Group | group 17 (halogens) | ||||||||||||||||||||
| Period | period 2 | ||||||||||||||||||||
| Block | p | ||||||||||||||||||||
| Electron configuration | [He] 2s2 2p5 | ||||||||||||||||||||
| Electrons per shell | 2, 7 | ||||||||||||||||||||
| Physical properties | |||||||||||||||||||||
| Phase at STP | gas | ||||||||||||||||||||
| Melting point | 53.53 K (−219.62 °C, −363.32 °F) | ||||||||||||||||||||
| Boiling point | 85.03 K (−188.12 °C, −306.62 °F) | ||||||||||||||||||||
| Density (at STP) | 1.696 g/L | ||||||||||||||||||||
| when liquid (at b.p.) | 1.505 g/cm3 | ||||||||||||||||||||
| Critical point | 144.4 K, 5.215 MPa | ||||||||||||||||||||
| Heat of vaporization | 6.51 kJ/mol | ||||||||||||||||||||
| Molar heat capacity | (Cp) (21.1 °C) 825 J·mol−1·K−1 (Cv) (21.1 °C) 610 J/(mol·K) |
||||||||||||||||||||
Vapor pressure
|
|||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||
| Oxidation states | −1 (oxidizes oxygen) | ||||||||||||||||||||
| Electronegativity | Pauling scale: 3.98 | ||||||||||||||||||||
| Ionization energies |
|
||||||||||||||||||||
| Covalent radius | 64 pm | ||||||||||||||||||||
| Van der Waals radius | 135 pm | ||||||||||||||||||||
| Spectral lines of fluorine | |||||||||||||||||||||
| Other properties | |||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||
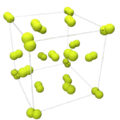
| Crystal structure | cubic
the structure shows solid fluorine, just under the melting point, 1 atm |
||||||||||||||||||||
| Thermal conductivity | 0.02591 W/(m⋅K) | ||||||||||||||||||||
| Magnetic ordering | diamagnetic | ||||||||||||||||||||
| CAS Number | 7782-41-4 | ||||||||||||||||||||
| History | |||||||||||||||||||||
| Discovery | André-Marie Ampère (1810) | ||||||||||||||||||||
| First isolation | Henri Moissan (June 26, 1886) | ||||||||||||||||||||
| Named by | Humphry Davy | ||||||||||||||||||||
| Main isotopes of fluorine | |||||||||||||||||||||
|
|||||||||||||||||||||
| reference | |||||||||||||||||||||
Fluorine (symbol F) is a special type of matter called a chemical element. It's known for being very reactive and can be dangerous. Its atomic number is 9, meaning it has 9 protons in its center. Fluorine is part of Group 17 on the periodic table, which are known as the halogens.
Contents
What Is Fluorine Like?
Fluorine is a light yellow gas. It is made of two fluorine atoms joined together, called a diatomic molecule. This element is the most reactive of all known elements. It really loves to grab electrons from other atoms. This makes it a very strong oxidizing agent.
Fluorine can even take electrons from water, which creates oxygen gas. It can also make propane burst into flames without needing a spark. Even metals can catch fire when exposed to fluorine gas. When fluorine reacts with other things, it forms a stable particle called a fluoride ion.
Temperature Points
Fluorine needs to be very cold to change its state. It melts at about -363.33°F (-219.62°C). It boils, turning into a gas, at about -306.62°F (-188.12°C).
Fluorine Compounds
Chemical compounds that contain fluorine are called fluorides. In these compounds, fluorine always has a special charge of -1. Some common examples of fluorine compounds include:
- Hydrofluoric acid: This is a strong acid made from hydrogen fluoride dissolved in water.
- Hydrogen fluoride: A gas that is very corrosive.
- Sodium fluoride: Often found in toothpaste.
- Sulfur hexafluoride: A gas used in many industrial applications.
- Tin(II) fluoride: Another compound sometimes used in dental products.
Where Is Fluorine Found?
You won't find pure fluorine as an element on Earth. It's far too reactive! Instead, it's always found combined with other elements in compounds called fluorides.
Many fluorides are found naturally in the Earth's crust. They are in rocks, coal, and clay. One important mineral that contains fluorine is fluorite.
How Is Fluorine Made?
Most fluorine is made using a process called electrolysis. This involves dissolving hydrogen fluoride in potassium fluoride. Then, an electric current is passed through this melted mixture. This process separates the hydrogen and fluorine. Hydrogen forms on one side, and fluorine forms on the other. It's very important to keep these two sides separate, or the setup could explode!
In 1986, a scientist managed to make fluorine without using electrolysis. They used different chemical compounds to create manganese(IV) fluoride, which then released fluorine gas.
What Is Fluorine Used For?
Fluorine and its compounds have many important uses:
- Nuclear Energy: Fluorine is used to prepare uranium for nuclear weapons and nuclear power.
- Industrial Gas: It helps make sulfur hexafluoride, a gas used in electrical equipment.
- Electronics: Fluorine is important in making integrated circuits, which are tiny parts of computers and phones.
- Everyday Products:
Safety Information
Pure fluorine gas is extremely reactive and very dangerous. It can react with almost anything, even glass. It is also highly poisonous.
Fluoride ions, which are found in fluorine compounds, are less dangerous but can still be toxic if too much is consumed. For example, eating too much toothpaste with fluoride could cause fluoride poisoning. However, fluoride ions are not as reactive as pure fluorine gas.
Related Topics
Images for kids
-
Steelmaking illustration from De re metallica
-
Chlorine trifluoride, whose corrosive potential ignites asbestos, concrete, sand and other fire retardants
-
Immiscible layers of colored water (top) and much denser perfluoroheptane (bottom) in a beaker; a goldfish and crab cannot penetrate the boundary; quarters rest at the bottom.
-
Fluorosurfactant-treated fabrics are often hydrophobic
-
Perfluorooctanesulfonic acid, a key Scotchgard component until 2000
See also
 In Spanish: Flúor para niños
In Spanish: Flúor para niños
 | William M. Jackson |
 | Juan E. Gilbert |
 | Neil deGrasse Tyson |