Sodium facts for kids
 |
||||||||||||||||||||||||||||
| Sodium | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance | silvery white metallic | |||||||||||||||||||||||||||
| Standard atomic weight Ar, std(Na) | 22.98976928(2) | |||||||||||||||||||||||||||
| Sodium in the periodic table | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Atomic number (Z) | 11 | |||||||||||||||||||||||||||
| Group | group 1: hydrogen and alkali metals | |||||||||||||||||||||||||||
| Period | period 3 | |||||||||||||||||||||||||||
| Block | s | |||||||||||||||||||||||||||
| Electron configuration | [Ne] 3s1 | |||||||||||||||||||||||||||
| Electrons per shell | 2,8,1 | |||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||
| Melting point | 370.87 K (97.72 °C, 207.9 °F) | |||||||||||||||||||||||||||
| Boiling point | 1156 K (883 °C, 1621 °F) | |||||||||||||||||||||||||||
| Density (near r.t.) | 0.968 g/cm3 | |||||||||||||||||||||||||||
| when liquid (at m.p.) | 0.927 g/cm3 | |||||||||||||||||||||||||||
| Critical point | (extrapolated) 2573 K, 35 MPa |
|||||||||||||||||||||||||||
| Heat of fusion | 2.60 kJ/mol | |||||||||||||||||||||||||||
| Heat of vaporization | 97.42 kJ/mol | |||||||||||||||||||||||||||
| Molar heat capacity | 28.230 J/(mol·K) | |||||||||||||||||||||||||||
Vapor pressure
|
||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||
| Oxidation states | −1, +1 (a strongly basic oxide) | |||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 0.93 | |||||||||||||||||||||||||||
| Ionization energies |
|
|||||||||||||||||||||||||||
| Atomic radius | empirical: 186 pm | |||||||||||||||||||||||||||
| Covalent radius | 166±9 pm | |||||||||||||||||||||||||||
| Van der Waals radius | 227 pm | |||||||||||||||||||||||||||
| Spectral lines of Sodium | ||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||||
| Crystal structure | body-centered cubic (bcc) | |||||||||||||||||||||||||||
| Speed of sound thin rod | 3200 m/s (at 20 °C) | |||||||||||||||||||||||||||
| Thermal expansion | 71 µm/(m⋅K) (at 25 °C) | |||||||||||||||||||||||||||
| Thermal conductivity | 142 W/(m⋅K) | |||||||||||||||||||||||||||
| Electrical resistivity | 47.7 n Ω⋅m (at 20 °C) | |||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | |||||||||||||||||||||||||||
| Young's modulus | 10 GPa | |||||||||||||||||||||||||||
| Shear modulus | 3.3 GPa | |||||||||||||||||||||||||||
| Bulk modulus | 6.3 GPa | |||||||||||||||||||||||||||
| Mohs hardness | 0.5 | |||||||||||||||||||||||||||
| Brinell hardness | 0.69 MPa | |||||||||||||||||||||||||||
| CAS Number | 7440-23-5 | |||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||
| Discovery | Humphry Davy (1807) | |||||||||||||||||||||||||||
| First isolation | Humphry Davy (1807) | |||||||||||||||||||||||||||
| Symbol | "Na": from New Latin natrium, coined from German Natron, 'natron' | |||||||||||||||||||||||||||
| Main isotopes of Sodium | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
Sodium is a chemical element with the symbol Na. Its name comes from the Latin word natrium. It has an atomic number of 11. Sodium is a soft, silvery-white, and very reactive metal.
It's an alkali metal, found in group 1 of the periodic table. This means it has one electron in its outer shell. Sodium easily gives this electron away to form a positively charged ion called Na+. The most common type of sodium is called Na-23.
You won't find pure sodium metal in nature. It's too reactive! Instead, it's always found combined with other elements in chemical compounds. Sodium is the sixth most common element in the Earth's crust. It's found in many minerals like feldspars and halite, which is also known as rock salt (NaCl).
Lots of sodium salts dissolve easily in water. Over millions of years, water has washed sodium ions out of Earth's minerals. That's why sodium and chlorine are the most common dissolved elements in the ocean.
Contents
Discovery and Naming
Sodium was first separated by Humphry Davy in 1807. He was an English scientist. He used a process called electrolysis to get it from sodium hydroxide. The name "sodium" comes from "soda," which is a common name for sodium hydroxide or sodium carbonate.
Properties of Sodium
Sodium is a light, silver-colored metal. It's so soft that you can easily cut it with a knife. When you cut it, the fresh surface looks shiny. But it quickly turns white because it reacts with the air. This reaction forms sodium hydroxide and sodium carbonate.
Sodium is a bit lighter than water. When it touches water, it floats and reacts very quickly. This reaction creates hydrogen gas and sodium hydroxide. The hydrogen gas can even catch fire! Because sodium melts at a low temperature, it often melts when it reacts with water. Sodium is very reactive because it has only one electron in its outer shell, which it easily loses.
Compared to other alkali metals, sodium is usually less reactive than potassium. However, it is more reactive than lithium.
Sodium in Compounds
Sodium almost always exists as a +1 oxidation state when it forms chemical compounds. This means it has lost one electron. Many compounds contain sodium ions. Here are some common ones:
- Sodium bicarbonate: This is also known as baking soda, used in cooking.
- Sodium carbonate: Used to make glass.
- Sodium chloride: This is common table salt that you use for food. It's also used to melt ice.
- Sodium fluoride: Often found in toothpastes to help prevent cavities.
- Sodium hydroxide: Also called lye, it's a very strong base used to make soap.
- Sodium hypochlorite: This is the main ingredient in bleach, used for cleaning and disinfecting.
Uses of Sodium
Pure Sodium Uses
Pure sodium metal is used in some special ways. It helps in making certain organic compounds. It's also used in the orange-colored street lights you might see.
Sodium Compound Uses
Sodium compounds are very important in our daily lives.
- They are used to make soaps.
- You'll find them in toothpaste.
- Sodium bicarbonate is used in baking.
- Some sodium compounds are in antacids, which help with stomach upset.
Where Sodium is Found
As mentioned, pure sodium metal is not found in nature because it's too reactive. It always exists as an ion in chemical compounds. Sodium ions are very common in the ocean, making seawater salty. It's also found as sodium chloride in the Earth's crust, where it can be mined.
Sodium metal is usually made by a process called electrolysis. This involves using electricity to separate sodium from very hot, melted sodium chloride.
Sodium in Living Things
Sodium ions, often in the form of sodium chloride, are very important for the human body and all animals. They help with many body functions. However, eating too much salt can cause health problems, so it's important to have a balanced diet. Many ocean creatures also rely on the right amount of sodium ions in seawater to survive.
Related Pages
- List of common elements
- Hyponatremia (a medical problem caused by not having enough sodium in the body)
Images for kids
-
The light emission spectrum for sodium, showing the bright yellow D line.
-
A positive flame test for sodium shows a bright yellow color.
-
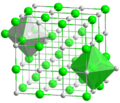
The structure of sodium chloride, showing how sodium and chlorine ions are arranged.
See also
 In Spanish: Sodio para niños
In Spanish: Sodio para niños
 | May Edward Chinn |
 | Rebecca Cole |
 | Alexa Canady |
 | Dorothy Lavinia Brown |