Hydrogen facts for kids

Purple glow in its plasma state
|
|||||||||||||||||||||
| Hydrogen | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance | colorless gas | ||||||||||||||||||||
| Standard atomic weight Ar, std(H) | [1.00784, 1.00811] conventional: 1.008 | ||||||||||||||||||||
| Hydrogen in the periodic table | |||||||||||||||||||||
|
|||||||||||||||||||||
| Atomic number (Z) | 1 | ||||||||||||||||||||
| Group | group 1: hydrogen and alkali metals | ||||||||||||||||||||
| Period | period 1 | ||||||||||||||||||||
| Block | s | ||||||||||||||||||||
| Electron configuration | 1s1 | ||||||||||||||||||||
| Electrons per shell | 1 | ||||||||||||||||||||
| Physical properties | |||||||||||||||||||||
| Phase at STP | gas | ||||||||||||||||||||
| Melting point | 13.99 K (−259.16 °C, −434.49 °F) | ||||||||||||||||||||
| Boiling point | 20.271 K (−252.879 °C, −423.182 °F) | ||||||||||||||||||||
| Density (at STP) | 0.08988 g/L | ||||||||||||||||||||
| when liquid (at m.p.) | 0.07 g/cm3 (solid: 0.0763 g/cm3) | ||||||||||||||||||||
| when liquid (at b.p.) | 0.07099 g/cm3 | ||||||||||||||||||||
| Triple point | 13.8033 K, 7.041 kPa | ||||||||||||||||||||
| Critical point | 32.938 K, 1.2858 MPa | ||||||||||||||||||||
| Heat of fusion | (H2) 0.117 kJ/mol | ||||||||||||||||||||
| Heat of vaporization | (H2) 0.904 kJ/mol | ||||||||||||||||||||
| Molar heat capacity | (H2) 28.836 J/(mol·K) | ||||||||||||||||||||
Vapor pressure
|
|||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||
| Oxidation states | −1, +1 (an amphoteric oxide) | ||||||||||||||||||||
| Electronegativity | Pauling scale: 2.20 | ||||||||||||||||||||
| Ionization energies |
|
||||||||||||||||||||
| Covalent radius | 31±5 pm | ||||||||||||||||||||
| Van der Waals radius | 120 pm | ||||||||||||||||||||
| Spectral lines of hydrogen | |||||||||||||||||||||
| Other properties | |||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||
| Crystal structure | hexagonal | ||||||||||||||||||||
| Speed of sound | 1310 m/s (gas, 27 °C) | ||||||||||||||||||||
| Thermal conductivity | 0.1805 W/(m⋅K) | ||||||||||||||||||||
| Magnetic ordering | diamagnetic | ||||||||||||||||||||
| Molar magnetic susceptibility | −3.98·10−6 cm3/mol (298 K) | ||||||||||||||||||||
| CAS Number | 12385-13-6 1333-74-0 (H2) |
||||||||||||||||||||
| History | |||||||||||||||||||||
| Discovery | Henry Cavendish (1766) | ||||||||||||||||||||
| Named by | Antoine Lavoisier (1783) | ||||||||||||||||||||
| Main isotopes of hydrogen | |||||||||||||||||||||
|
|||||||||||||||||||||
Hydrogen is a chemical element. It has the symbol H and atomic number 1. It has a standard atomic weight of 1.008, meaning it is the lightest element in the periodic table.
Hydrogen is the most common chemical element in the Universe, making up 75% of all normal (baryonic) matter (by mass). Most stars are mostly hydrogen. Hydrogen's most common isotope has one proton with one electron orbiting around it.
Contents
Properties
Hydrogen is classed as a reactive nonmetal, unlike the other elements appearing in the first column of the periodic table, which are classed alkali metals. The solid form of hydrogen is expected to behave like a metal, however.
When alone, hydrogen usually binds with itself to make dihydrogen (H2) which is very stable, due to its high bond dissociation energy of 435.7 kJ/mol. At standard temperature and pressure, this hydrogen gas (H2) has no colour, smell or taste. It is not toxic. It is a nonmetal and burns very easily.
Combustion
Molecular hydrogen is flammable and reacts with oxygen:
2 H2(g) + O2(g) → 2 H2O(l) + 572 kJ (286 kJ/mol)
At temperatures above 500 degrees Celsius, hydrogen spontaneously ignites in air.
Compounds
While hydrogen gas in its pure form is not reactive, it does form compounds with many elements, particularly halogens, which are very electronegative. Hydrogen also forms vast arrays with carbon atoms, forming hydrocarbons. The study of the properties of hydrocarbons are known as organic chemistry.
The H- anion (negatively charged atom) is called a hydride, although the term is not widely used. An example of a hydride is lithium hydride (LiH), which is used as a "spark plug" in nuclear weapons.
Acids
Acids dissolved in water typically contain high levels of hydrogen ions, in other words, free protons. The level of them is usually used to determine its pH, which basically means the content of hydrogen ions in a particular volume. For example, hydrochloric acid, found in people's stomachs, can dissociate into a chloride anion and a free proton, and the property of the free proton is how it can digest food by corroding it.
Although rare on Earth, the H3+ cation is one of the most common ions in the universe.
Isotopes
- Main article: isotopes of hydrogen
Hydrogen has 7 known isotopes, two of which are stable (1H and 2H), which are commonly referred to protium and deuterium. The isotope 3H is known as tritium and has a half life of 12.33 years, and is produced in small amounts by cosmic rays. The remaining 4 isotopes have half lives on the scale of yoctoseconds.
Hydrogen in nature
In its pure form on Earth, hydrogen is usually a gas. Hydrogen is also one of the parts that make up a water molecule. Hydrogen is important because it is the fuel that powers the Sun and other stars. Hydrogen makes up about 74% of the entire universe. Hydrogen's symbol on the Periodic Table of Elements is H.
Pure hydrogen is normally made of two hydrogen atoms connected together. Scientists call these diatomic molecules. Hydrogen will have a chemical reaction when mixed with most other elements. It has no color or smell.
Pure hydrogen is very uncommon in the Earth's atmosphere, because nearly all primordial hydrogen would have escaped into space due to its weight. In nature, it is usually in water. Hydrogen is also in all living things, as a part of the organic compounds that living things are made of. In addition, hydrogen atoms can combine with carbon atoms to form hydrocarbons. Petroleum and other fossil fuels are made of these hydrocarbons and commonly used to create energy for human use.
Some other facts about hydrogen:
- It is a gas at room temperature
- It acts like a metal when it is solid.
- It is the lightest element in the Universe.
- It is the most common element in the Universe.
- It burns or explodes when it touches a flame.
- glows purple when it is in plasma state.
History of Hydrogen
Hydrogen was first separated in 1671 by Robert Boyle. Henry Cavendish in 1776 identified it as a distinct element and discovered that burning it made water.
Antoine Lavoisier gave Hydrogen its name, from the Greek word for water, 'υδορ (pronounced /HEEW-dor/) and gennen meaning to "generate" as it forms water in a chemical reaction with oxygen.
Uses of Hydrogen
The main uses are in the petroleum industry and in making ammonia by the Haber process. Some is used elsewhere in the chemical industry. A little of it is used as fuel, for example in rockets for spacecraft. Most of the hydrogen that people use comes from a chemical reaction between natural gas and steam.
Nuclear fusion
Nuclear fusion is a very powerful source of energy. It relies on forcing atoms together to make helium and energy, exactly as happens in a star like the Sun, or in a hydrogen bomb. This needs a large amount of energy to get started, and is not easy to do yet. A big advantage over nuclear fission, which is used in today's nuclear power stations, is that it makes less nuclear waste and does not use a toxic and rare fuel like uranium. More than 600 million tons of hydrogen undergo fusion every second on the Sun.
Using hydrogen
Hydrogen is mostly used in the petroleum industry, to change heavy petroleum fractions into lighter, more useful ones. It is also used to make ammonia. Smaller amounts are burned as fuel. Most hydrogen is made by a reaction between natural gas and steam.
The electrolysis of water breaks water into hydrogen and oxygen, using electricity. Burning hydrogen combines with oxygen molecules to make steam (pure water vapor). A fuel cell combines hydrogen with an oxygen molecule, releasing an electron as electricity. For these reasons, many people believe hydrogen power will eventually replace other synthetic fuels.
Hydrogen can also be burned to make heat for steam turbines or internal combustion engines. Like other synthetic fuels, hydrogen can be created from natural fuels such as coal or natural gas, or from electricity, and therefore represents a valuable addition to the power grid; in the same role as natural gas. Such a grid and infrastructure with fuel cell vehicles is now planned by a number of countries including Japan, Korea and many European countries. This allows these countries to buy less petroleum, which is an economic advantage. The other advantage is that used in a fuel cell or burned in a combustion engine as in a hydrogen car, the motor does not make pollution. Only water, and a small amount of nitrogen oxides, forms.
| Periodic table | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
Images for kids
-
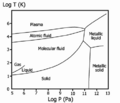
Phase diagram of hydrogen. The temperature and pressure scales are logarithmic, so one unit corresponds to a 10x change. The left edge corresponds to 105 Pa, which is about atmospheric pressure.
-
NGC 604, a giant region of ionized hydrogen in the Triangulum Galaxy
-
Illustrating inputs and outputs of methane pyrolysis, a process to produce hydrogen
See also
 In Spanish: Hidrógeno para niños
In Spanish: Hidrógeno para niños