History of chemistry facts for kids

The history of chemistry covers a long time, from ancient times until today. By 1000 BC, people were already using skills that would become parts of chemistry. For example, they discovered fire. They also learned to get metals from ores. People made pottery and glazes. They fermented beer and wine. They took chemicals from plants for medicine and perfume. They turned fat into soap. They made glass and alloys like bronze.
Before modern chemistry, there was a practice called alchemy. Alchemists tried to understand matter and how it changed. They were not always successful in explaining things. However, they did many experiments and wrote down their results. This helped set the stage for modern chemistry. Both alchemy and chemistry study matter and its changes. But chemists use the scientific method in their work. The history of chemistry is also linked to the history of thermodynamics. This is especially true through the work of Willard Gibbs.
Contents
- Ancient Discoveries
- Medieval Alchemy
- 17th and 18th Centuries: Early Chemistry
- 19th Century Chemistry
- 20th Century Chemistry
- Mathematics and Chemistry
- What Chemistry Studies
- See also
Ancient Discoveries
Early Humans and Chemistry
Scientists found a workshop from 100,000 years ago in Blombos Cave, South Africa. It showed that early humans knew some basic chemistry. They processed a red earth material called ochre. Paintings on cave walls also show early humans mixing animal blood with other liquids. This suggests they had a small understanding of chemistry.
First Uses of Metals
The first metal humans used was likely gold. You can find gold naturally as "native" gold. Small amounts of natural gold were found in Spanish caves from around 40,000 BC.
Silver, copper, tin, and meteoric iron also exist naturally. This allowed ancient cultures to do some metalworking. Egyptian weapons made from meteoric iron around 3000 BC were very special. People called them "daggers from Heaven."
Fire was probably the first chemical reaction humans controlled. For thousands of years, fire seemed like a magical force. It could change wood into ash or boil water. Fire was important for many parts of early life. It helped with cooking and heating homes. It also helped make pottery, bricks, and melt metals for tools. Fire led to the discovery of glass. It also led to cleaning metals, which started metallurgy. People looked for ways to purify metals. Gold, known in ancient Egypt by 2900 BC, became a very valuable metal.
The Bronze Age
Some metals can be taken from their ores just by heating the rocks in a fire. These include tin, lead, and (at higher heat) copper. This process is called smelting. The first signs of this metal extraction are from 6,000 to 5,000 BC. They were found in Serbia. The earliest copper smelting is from 5500 BC. Other early metal signs are from 3,000 BC in places like Portugal, Spain, and the UK. It is hard to know the exact start of these things. New discoveries are always happening.
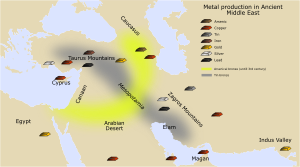
The first metals were single elements or natural mixtures. But by mixing copper and tin, people made a better metal called bronze. This was a huge step forward. It started the Bronze Age around 3500 BC. During this time, people learned to smelt copper and tin from their ores. Then they cast bronze. These natural ores often had arsenic in them. Copper/tin ores were rare. This is why tin bronzes were not common in western Asia before 3000 BC. After the Bronze Age, armies wanted better weapons. Countries in Eurasia became strong by making better alloys. These alloys led to better armor and weapons. India also made great progress in metallurgy and alchemy.
The Iron Age
Getting iron from its ore is much harder than getting copper or tin. Iron was not better than bronze for tools until steel was discovered. But iron ore is much more common than copper or tin. So, it was often available locally. This meant people did not need to trade for it.
The Hittites likely invented iron working around 1200 BC. This started the Iron Age. Knowing how to get and work iron was key to the success of the Philistines. The Iron Age refers to when iron working (ferrous metallurgy) began. Many ancient cultures and empires developed iron working. These include the Middle East, ancient Iran, Egypt, Nubia, Anatolia (Turkey), ancient Nok, Carthage, the Greeks, and Romans. Medieval Europe, China, India, and Japan also made progress. Ancient China made many advances in metallurgy. They invented the blast furnace, cast iron, and water-powered hammers. They also made double-acting piston bellows.
Ancient Ideas About Atoms

People in ancient times wondered why different things had different properties. Why were some colorful, dense, or smelly? Why did they exist as gas, liquid, or solid? Why did they react differently to water, fire, or heat? This led ancient thinkers to create the first theories about nature and chemistry. Every ancient civilization probably had such ideas. They all tried to find a few basic classical elements that made up everything. Things like air, water, earth, fire, and light were common ideas. Even abstract ideas like thoughts or heaven were included. For example, ancient Greek, Indian, Mayan, and Chinese philosophies all saw air, water, earth, and fire as main elements.
Around 420 BC, Empedocles said that all matter was made of these four elements. The early idea of atomism came from ancient Greece and ancient India. The Greek thinker Democritus made Greek atomism popular. Around 380 BC, he said that matter was made of tiny, unbreakable particles called "atomos." Earlier, Leucippus also said atoms were the smallest part of matter. At the same time, the Indian philosopher Kanada said something similar in his writings. Aristotle disagreed with the idea of atoms in 330 BC. A Greek text from around 380 BC said the human body was made of four "humours." Epicurus (around 300 BC) believed in a universe of unbreakable atoms. He thought people were responsible for living a balanced life.
Around 50 BC, the Roman poet Lucretius wrote De rerum natura (The Nature of Things). He wanted to explain Epicurean ideas to Romans. In his work, Lucretius talked about atomism. He also explained the mind, soul, senses, and how the world developed.
The first alchemists in the Western world were from Greco-Roman Egypt in the first centuries AD. They did technical work and invented chemical tools. The bain-marie (water bath) is named after Mary the Jewess. Her work also describes the tribikos and kerotakis. Cleopatra the Alchemist described furnaces. She is also credited with inventing the alembic. Later, Zosimos of Panopolis wrote books on alchemy. He called them cheirokmeta, meaning "things made by hand." These books had many recipes and procedures. They also described instruments. Much of the early work on cleaning methods was described by Pliny the Elder. He wrote about them in his Naturalis Historia. He tried to explain these methods. He also made good observations about many minerals.
Medieval Alchemy

The system of elements used in medieval alchemy came mainly from the Persian-Arab alchemist Jābir ibn Hayyān. It was based on the Greek idea of classical elements. His system had the four Aristotelian elements: air, earth, fire, and water. It also had two philosophical elements: sulphur (for burning) and mercury (for metallic properties). Early alchemists saw these as perfect forms of the universe's basic parts.
Later, the Swiss alchemist Paracelsus added a third principle: salt (for solidity). He called these three tria prima. Paracelsus thought Aristotle's four elements appeared as these three principles in things. He explained this by how wood burns. Mercury was the part that held the wood together. When it left (as smoke), the wood fell apart. Smoke showed volatility (mercury). Flames showed flammability (sulphur). The ash left behind showed solidity (salt).
The Philosopher's Stone
Alchemy is known for the search for the philosopher's stone. This search was full of symbolic mystery. It was very different from modern science. Alchemists worked to make changes on a spiritual or practical level. The practical side of alchemy greatly helped chemistry grow in Greco-Roman Egypt, the Islamic Golden Age, and then in Europe. Alchemy and chemistry both study what matter is made of and its properties. Until the 18th century, they were not separate subjects. The word chymistry was used for the mix of alchemy and chemistry before that time.
During the Renaissance, practical alchemy stayed popular. It was called iatrochemistry (medical chemistry). Spiritual alchemy also grew. It went back to its older roots. So, the search for the philosopher's stone was not replaced by science. Respected scientists and doctors still worked on it until the early 18th century. Famous early modern alchemists who helped science include Jan Baptist van Helmont, Robert Boyle, and Isaac Newton.
Alchemy in the Islamic World
In the Islamic World, Muslim scholars translated ancient Greek and Hellenistic writings into Arabic. They also experimented with scientific ideas. Arabic writings from the 8th-century alchemist Jābir ibn Hayyān sorted chemical substances. They also showed how to make an inorganic compound (ammonium chloride) from organic things. Some of Jabir's works were translated into Latin. In 13th-century Europe, a writer called pseudo-Geber wrote alchemical and metalworking texts under this name. Later Muslim thinkers like Abū al-Rayhān al-Bīrūnī and Avicenna disagreed with alchemy's theories. They especially doubted the idea of turning metals into gold.
Problems with Alchemy
Alchemy had several problems when we look at it today. There was no clear way to name new compounds. The language was secret and unclear. Words meant different things to different people. As one history book says: "The language of alchemy soon developed a secret vocabulary. It was meant to hide information from those who weren't part of it. Today, we can barely understand this language."
Writers like Geoffery Chaucer and Ben Jonson even made fun of alchemy's confusing language. Chaucer's story showed the dishonest side of alchemy. People would try to make fake gold from cheap materials. Even earlier, Dante Alighieri knew about this fraud. He put alchemists in his Inferno. In 1317, Pope John XXII ordered all alchemists to leave France for making fake money. England made a law in 1403. It said that "multiplying metals" (making fake gold) could be punished by death. Despite these strict rules, alchemy did not disappear. Kings and rich people still wanted to find the philosopher's stone and the elixir of life.
There was also no agreed-upon scientific method. Experiments could not be easily repeated by others. Results were not reported in clear language. Alchemists often included useless information in their methods. Things like the timing of tides or moon phases were mentioned. The secret nature of alchemy helped hide that they weren't sure about much at all. By the 14th century, people started to doubt alchemy. It became clear that a scientific method was needed. Experiments had to be repeatable. Results had to be clear about what was known and unknown.
17th and 18th Centuries: Early Chemistry
Practical efforts to improve how ores were refined and metals were extracted helped early chemists in the 16th century. One such person was Georg Agricola (1494–1555). He published his great work De re metallica in 1556. His book described the complex ways of mining metal ores, extracting metals, and metallurgy of his time. He took away the mystery from the subject. This created a practical base for others to build upon. His work described many types of furnaces used to smelt ore. It also made people more interested in minerals and what they were made of. Agricola is known as the "father of metallurgy."
In 1605, Sir Francis Bacon wrote about what would later be called the scientific method. In the same year, Michal Sedziwój wrote an alchemy book. It suggested that "food of life" existed in the air. This was later found to be oxygen. In 1615, Jean Beguin published Tyrocinium Chymicum. This was an early chemistry textbook. It showed the first ever chemical equation. In 1637, René Descartes wrote Discours de la méthode. It included an outline of the scientific method.
The Dutch chemist Jan Baptist van Helmont's book Ortus medicinae was published after he died in 1648. Some say this book was a key step between alchemy and chemistry. It greatly influenced Robert Boyle. The book has many experiment results. It also set up an early version of the law of conservation of mass. Van Helmont worked after Paracelsus. He suggested there were substances other than air. He called them "gas" from the Greek word chaos. He did several experiments with gases. Van Helmont is also known for his ideas on spontaneous generation. He also did a famous 5-year tree experiment. He is seen as the founder of pneumatic chemistry.
Robert Boyle

The Anglo-Irish chemist Robert Boyle (1627–1691) is thought to have started the separation of chemistry from alchemy. Boyle doubted the idea of elements. He also believed in alchemy. But he was important in making chemistry an independent and philosophical subject. He is most famous for Boyle's law, which he shared in 1662. This law describes how the pressure and volume of a gas are related. If temperature stays the same, as one goes up, the other goes down.
Boyle is also known for his important book The Sceptical Chymist (1661). In it, he urged chemists to experiment carefully. Boyle questioned common alchemical ideas. He argued that scientists should be more "philosophical" and less focused on making money. He rejected the old idea of four elements: earth, fire, air, and water. He suggested a new idea of atoms and chemical reactions. These could be studied with strict experiments.
Boyle also tried to purify chemicals. This helped him get results that could be repeated. He strongly supported the idea that physical properties and interactions of matter could be explained by mechanics. Boyle believed in atoms. But he preferred the word corpuscle. He said that the smallest part of matter that still keeps its properties is a corpuscle.
Boyle repeated van Helmont's tree experiment. He was the first to use indicators. These changed colors with acidity. He also did many studies with an air pump. He noticed that the mercury in a barometer fell as air was pumped out. He also saw that pumping air out of a container would put out a flame. It would also kill small animals inside. Boyle's work helped build the foundation for the Chemical Revolution much later.
Phlogiston Theory: Rise and Fall

In 1702, German chemist Georg Stahl named "phlogiston". This was believed to be a substance released during burning. Around 1735, Swedish chemist Georg Brandt studied a dark blue color in copper ore. Brandt showed that this color came from a new element, later called cobalt. In 1751, Axel Fredrik Cronstedt, a student of Stahl's, found another new metallic element in copper ore. He named it nickel. Cronstedt is one of the founders of modern mineralogy. He also found the mineral scheelite in 1751. He called it tungsten, meaning "heavy stone" in Swedish.
In 1754, Scottish chemist Joseph Black isolated carbon dioxide. He called it "fixed air." In 1757, Louis Claude Cadet de Gassicourt made Cadet's fuming liquid. This was the first synthetic organometallic compound. In 1758, Joseph Black explained latent heat. This helped understand heat changes in phase changes. In 1766, English chemist Henry Cavendish isolated hydrogen. He called it "inflammable air." Cavendish found hydrogen was a colorless, odorless gas that burns. It could form an explosive mix with air. He published that burning hydrogen in "dephlogisticated air" (oxygen) made water.
In 1773, Swedish chemist Carl Wilhelm Scheele discovered oxygen. He called it "fire air." But he did not publish his discovery right away. In 1774, English chemist Joseph Priestley also isolated oxygen. He called it "dephlogisticated air." Priestley published his work before Scheele. Priestley was famous for inventing soda water. He also wrote about electricity. And he discovered several gases, especially oxygen. However, Priestley strongly defended the phlogiston theory. He rejected the ideas that led to the chemical revolution. This left him alone in the scientific community.
In 1781, Carl Wilhelm Scheele found a new acid, tungstic acid. It could be made from Cronstedt's scheelite. Scheele and Torbern Bergman thought a new metal could be made from this acid. In 1783, José and Fausto Elhuyar found an acid from wolframite that was the same as tungstic acid. Later that year, in Spain, they successfully isolated the metal now called tungsten. They did this by using charcoal to reduce the acid. They are credited with discovering the element.
Volta and the First Battery
Italian physicist Alessandro Volta built a device to gather a large electrical charge. He studied "animal electricity" discovered by Luigi Galvani in the 1780s. Volta found that electric current came from different metals touching. The frog leg was just a detector. In 1794, Volta showed that two metals and a brine-soaked cloth could make an electric current.
In 1800, Volta stacked pairs of copper (or silver) and zinc discs. He separated them with cloth soaked in brine. This made the liquid conduct electricity better. When the top and bottom were connected by a wire, an electric current flowed. This was the first electrical battery to make electricity.
Volta is seen as the founder of electrochemistry. A Galvanic cell (or voltaic cell) makes electrical energy from a natural chemical reaction. It usually has two different metals connected by a salt bridge.
Antoine-Laurent de Lavoisier

Antoine-Laurent de Lavoisier showed that water could not turn into earth. He proved that the sediment from boiling water came from the container. He burned phosphorus and sulfur in air. He showed that the products weighed more than the original samples. The extra mass came from the air. So, in 1789, he created the Law of Conservation of Mass. This is also called "Lavoisier's Law."
Lavoisier repeated Priestley's experiments. He showed that air has two parts. One part combines with metals to form calxes. In 1778, he showed that the "air" causing burning was also the source of acidity. The next year, he named this part oxygen (Greek for acid-former). He named the other part azote (Greek for no life). Because he described it so well, Lavoisier is also credited with discovering oxygen, along with Priestley and Scheele. He also found that Cavendish's "inflammable air" (which he called hydrogen, Greek for water-former) combined with oxygen to make water. In 1783, Lavoisier showed that the phlogiston theory of burning was wrong. Mikhail Lomonosov also worked on chemistry in Russia. He also rejected the phlogiston theory. He had ideas similar to the kinetic theory of gases. Lomonosov thought heat was a form of motion. He also stated the idea of conservation of matter.
Lavoisier worked with Claude Louis Berthollet and others. They created a system for naming chemicals. This system is the basis of how we name chemical compounds today. In his 1787 book, Lavoisier invented the naming and classification system. It included names like sulfuric acid, sulfates, and sulfites. In 1785, Berthollet was the first to use chlorine gas as a bleach. In the same year, he found what the gas ammonia was made of. Berthollet first made a modern bleaching liquid in 1789. He did this by passing chlorine gas through a solution of sodium carbonate. This made a weak solution of sodium hypochlorite. He also studied and first made potassium chlorate (KClO3), a strong chlorine bleach. This is known as Berthollet's Salt. Berthollet also helped with the theory of chemical equilibrium. He studied how reversible reactions work.
Lavoisier's Traité Élémentaire de Chimie (Elementary Treatise of Chemistry, 1789) was the first modern chemistry textbook. It presented new chemistry ideas in a clear way. It clearly stated the Law of Conservation of Mass. It also said that phlogiston did not exist. It listed elements, or substances that could not be broken down. These included oxygen, nitrogen, hydrogen, phosphorus, mercury, zinc, and sulfur. But his list also included light and caloric. He thought these were materials. Lavoisier stressed that his chemistry was based on observation. He said, "I have tried...to find the truth by connecting facts. I tried to use reasoning as little as possible. Reasoning is often unreliable and tricks us. I wanted to follow the light of observation and experiment as much as possible." However, he believed that atoms could not truly exist. Lavoisier showed that living things take apart and rebuild air in the same way a burning body does.
With Pierre-Simon Laplace, Lavoisier used a calorimeter to measure the heat produced per unit of carbon dioxide. They found the same ratio for a flame and animals. This showed that animals produced energy by a type of burning. Lavoisier believed in the radical theory. This said that radicals, which act as a single group in a chemical reaction, would combine with oxygen. He thought all acids contained oxygen. He also found that diamond is a form of carbon.
Many of Lavoisier's helpers were important for chemistry. But his wife, Marie-Anne Lavoisier, was perhaps the most important. After they married, Marie-Anne studied chemistry, English, and drawing. She helped her husband by translating papers into English (a language he didn't know). She also kept records and drew the equipment he used. Because she could read and translate articles from Britain, Lavoisier knew about many chemical advances outside his lab. Marie-Anne also kept records of his work and made sure his books were published. Marie-Anne showed her skill as a chemist when she translated a book by Richard Kirwan. She found and corrected many errors. When she showed her translation and notes to Lavoisier, her contributions led to his rejection of the phlogiston theory.
Lavoisier made many basic contributions to chemistry. After his work, chemistry became a strict, quantitative science. This allowed for reliable predictions. The chemical revolution he started was a result of his effort to fit all experiments into one theory. He made sure chemists always used the chemical balance. He used oxygen to prove the phlogiston theory wrong. He also created a new system for naming chemicals. His further contributions were cut short. Lavoisier was executed during the French Revolution.
19th Century Chemistry
In 1802, French-American chemist Éleuthère Irénée du Pont started a gunpowder company in Delaware. He had learned about making gunpowder from Antoine Lavoisier. The French Revolution forced his family to move to the United States. Du Pont wanted to make the best powder. He was very careful about the quality of his materials. For 32 years, du Pont was president of E. I. du Pont de Nemours and Company. It grew into a very large and successful company in America.
Throughout the 19th century, chemists were divided. Some followed the atomic theory of John Dalton. Others, like Wilhelm Ostwald and Ernst Mach, did not. Even though supporters of atomic theory like Amedeo Avogadro and Ludwig Boltzmann made great progress in explaining how gases behave, this argument was not settled. It was finally settled by Jean Perrin's experiments on Einstein's explanation of Brownian motion in the early 1900s.
Before the argument was settled, many had already used the idea of atoms in chemistry. A big example was Svante Arrhenius's ion theory. It had ideas about the inside of atoms that were fully developed only in the 20th century. Michael Faraday was another early worker. His main contribution to chemistry was electrochemistry. He showed that a certain amount of electricity during electrolysis was linked to certain amounts of chemical elements. This meant elements combined in specific ratios. These findings, like Dalton's combining ratios, were early clues that matter was made of atoms.
John Dalton's Atomic Ideas

In 1803, English meteorologist and chemist John Dalton proposed Dalton's law. This law describes how parts of a gas mixture relate to the total pressure. He discovered this idea in 1801. It is also called Dalton's law of partial pressures.
Dalton also suggested a modern atomic theory in 1803. It said that all matter is made of small, unbreakable particles called atoms. Atoms of a certain element have unique features and weight. He said there were three types of atoms: simple (elements), compound (simple molecules), and complex (complex molecules). In 1808, Dalton published New System of Chemical Philosophy. In it, he gave the first modern scientific description of atomic theory. This work said that chemical elements are a specific type of atom. This rejected Newton's idea of chemical attractions.
Instead, Dalton figured out the amounts of elements in compounds. He did this by looking at the weights of the reacting substances. He set the atomic weight of hydrogen as one. Following Jeremias Benjamin Richter, he suggested that chemical elements combine in simple whole number ratios. This is called the law of multiple proportions or Dalton's law. Dalton clearly described this law in his book. The law of multiple proportions is a basic law of stoichiometry. It helped establish atomic theory. Even though his work was important for seeing atoms as real things and for chemical symbols, his book spent almost as much space on the caloric theory as on atomism.
French chemist Joseph Proust proposed the law of definite proportions. This law says that elements always combine in small, whole number ratios to form compounds. He based this on experiments from 1797 to 1804. Along with the law of multiple proportions, this law forms the basis of stoichiometry. These laws do not prove atoms exist. But they are hard to explain without assuming that chemical compounds are formed when atoms combine in constant proportions.
Jöns Jacob Berzelius

Jöns Jacob Berzelius was a Swedish chemist and a student of Dalton. He started a careful program to make accurate measurements and ensure chemicals were pure. Along with Lavoisier, Boyle, and Dalton, Berzelius is known as a father of modern chemistry. In 1828, he made a table of relative atomic weights. He used oxygen as a standard, setting its weight at 100. This table included all known elements. His work supported Dalton's atomic theory. It showed that inorganic chemical compounds are made of atoms combined in whole number amounts. He found the exact parts of many compounds. His results strongly supported Proust's Law of Definite Proportions. Berzelius also found that atomic weights are not always exact multiples of hydrogen's weight. This proved Prout's hypothesis wrong.
Because of his many atomic weight measurements, he created the classic system of chemical symbols and notation. He published this in his 1808 book Lärbok i Kemien. In it, elements were shortened to one or two letters from their Latin names. This system, like O for oxygen or Fe for iron, with numbers for amounts, is still used today. The only difference is that Berzelius used a superscript (H2O) instead of a subscript (H2O). Berzelius is credited with finding the elements silicon, selenium, thorium, and cerium. Students in his lab also found lithium and vanadium.
Berzelius developed the radical theory of chemical combination. This theory said that reactions happen when stable groups of atoms, called radicals, are exchanged between molecules. He believed that salts are compounds made of acids and bases. He found that the negative parts in acids were attracted to a positive electrode. The positive parts in a base were attracted to a negative electrode. Berzelius did not believe in the Vitalism Theory. Instead, he believed in a force that organized tissues in living things. Berzelius is also credited with creating the chemical terms "catalysis", "polymer", "isomer", and "allotrope". But his original definitions are very different from how we use them today. For example, he used "polymer" in 1833 for organic compounds with the same formula but different total weights.
New Elements and Gas Laws

English chemist Humphry Davy was a pioneer in electrolysis. He used Alessandro Volta's battery to break down common compounds. This helped him isolate new elements. He electrolyzed melted salts. He discovered several new metals, especially sodium and potassium. These are very reactive elements called alkali metals. Potassium was the first metal found by electrolysis. Davy discovered it in 1807 from potassium hydroxide. Before the 19th century, potassium and sodium were not seen as different. Davy isolated sodium in the same year. He did this by passing electric current through melted sodium hydroxide. When Davy heard that others made calcium amalgam, he tried it. Davy succeeded and found calcium in 1808. He did this by electrolyzing a mix of lime and mercuric oxide. He worked with electrolysis his whole life. In 1808, he also isolated magnesium, strontium, and barium.
Davy also experimented with gases by breathing them in. This was very dangerous and almost killed him several times. But it led to the discovery of the strange effects of nitrous oxide, known as laughing gas. Chlorine was found in 1774 by Swedish chemist Carl Wilhelm Scheele. He called it "dephlogisticated marine acid." He mistakenly thought it had oxygen. Scheele saw many properties of chlorine gas. It bleached litmus, killed insects, was yellow-green, and smelled like aqua regia. But Scheele could not publish his findings then. In 1810, Humphry Davy gave chlorine its current name (from the Greek word for green). He insisted that chlorine was an element. He also showed that oxygen could not be gotten from oxymuriatic acid (HCl solution). This discovery overturned Lavoisier's idea that acids were compounds of oxygen. Davy was a popular speaker and a good experimenter.

French chemist Joseph Louis Gay-Lussac was interested in studying gases quantitatively. In 1801–1802, he found that equal volumes of all gases expand equally with the same temperature increase. This is usually called "Charles's law." Gay-Lussac gave credit to Jacques Charles, who had similar ideas but did not publish them. John Dalton also found this law by 1801, but Gay-Lussac's description was more complete. In 1804, Gay-Lussac made daring balloon flights over 7,000 meters high. He used hydrogen-filled balloons. This allowed him to study gases more. He took magnetic measurements, pressure, temperature, and humidity readings. He also collected air samples to analyze later.
In 1808, Gay-Lussac announced his greatest achievement. From his and others' experiments, he found that gases at constant temperature and pressure combine in simple numerical proportions by volume. If the products were gases, their volumes also had a simple ratio to the reactants. This means gases react in small whole number volume ratios under the same conditions. This became known as "Gay-Lussac's law" or the "Law of Combining Volumes." With his colleague Louis Jacques Thénard, Gay-Lussac also did early electrochemistry research. They studied elements found by this method. They broke down boric acid using melted potassium. This led to the discovery of the element boron. They also helped in debates that changed Lavoisier's definition of acids. They also continued his work on analyzing organic compounds for oxygen and hydrogen.
French chemist Bernard Courtois discovered the element iodine in 1811. Courtois gave samples to his friends, Charles Bernard Desormes and Nicolas Clément, to continue research. He also gave some to Gay-Lussac and physicist André-Marie Ampère. On December 6, 1813, Gay-Lussac announced that the new substance was either an element or an oxygen compound. Gay-Lussac suggested the name "iode", from the Greek word for violet (because of the color of iodine vapor). Ampère had given some of his sample to Humphry Davy. Davy experimented with the substance and saw it was similar to chlorine. Davy sent a letter on December 10 to the Royal Society of London. He said he had found a new element. Arguments started between Davy and Gay-Lussac over who found iodine first. But both scientists agreed Courtois was the first to isolate it.
In 1815, Humphry Davy invented the Davy lamp. This allowed miners to work safely in coal mines with flammable gases. Many mining explosions happened because of firedamp or methane. These were often ignited by the open flames of miners' lamps. Davy thought of using an iron screen to enclose a lamp's flame. This would stop the methane burning inside the lamp from spreading outside. The idea of a safety lamp had been shown by others before. But Davy's use of wire gauze was used by many inventors in their later designs. Davy refused to patent the lamp. He received an award for his invention in 1816.

After Dalton published his atomic theory in 1808, most chemists quickly adopted some of his main ideas. But for 50 years, there was confusion about how to use atomic theory. Chemists in different countries had different systems for atoms. A paper in 1811 by Italian physicist Amedeo Avogadro (1776–1856) offered a solution. He suggested that equal volumes of gases at the same temperature and pressure have equal numbers of molecules. From this, it followed that the relative molecular weights of any two gases are the same as the ratio of their densities. Avogadro also thought that simple gases were not just single atoms. Instead, they were molecules of two or more atoms. This helped solve a problem Dalton and others had. Gay-Lussac had reported that above 100 °C, the volume of water vapor was twice the volume of oxygen used to form it. According to Avogadro, the oxygen molecule split into two atoms when forming water vapor.
Avogadro's idea was ignored for 50 years. Many reasons are given for this. There were some theory problems. For example, Jöns Jacob Berzelius's "dualism" said compounds were held by positive and negative electrical charges. This made it hard to imagine a molecule like oxygen with two similar atoms. Also, many chemists did not want to use physical methods to solve their problems. But by the mid-1800s, some important chemists felt the many different atomic weight systems were too confusing. Also, chemical evidence started to suggest Avogadro's idea might be right. In the 1850s, younger chemists like Alexander Williamson, Charles Gerhardt, Charles-Adolphe Wurtz, and August Kekulé started to push for changes in chemistry theory to match Avogadro's ideas.
Wöhler and the Vitalism Debate
In 1825, Friedrich Wöhler and Justus von Liebig were the first to find and explain isomers. These had been named earlier by Berzelius. They worked with cyanic acid and fulminic acid. They correctly figured out that isomerism was caused by atoms being arranged differently in a molecule. In 1827, William Prout put biomolecules into their modern groups: carbohydrates, proteins, and lipids. After people understood how burning worked, a debate started about vitalism. This was the idea that organic and inorganic substances were fundamentally different. The vitalism question changed greatly in 1828. Friedrich Wöhler made urea in the lab. This showed that organic compounds could be made from inorganic materials. This disproved the vitalism theory.
This opened a new area of research in chemistry. By the end of the 19th century, scientists could make hundreds of organic compounds. Important ones included mauve, magenta, and other synthetic dyes. The widely used drug aspirin was also made. The discovery of artificial urea making helped the theory of isomerism. This is because urea and ammonium cyanate have the same chemical formula. In 1832, Friedrich Wöhler and Justus von Liebig found and explained functional groups and radicals in organic chemistry. They also first made benzaldehyde. Liebig, a German chemist, made big contributions to agricultural and biological chemistry. He also helped organize organic chemistry. Liebig is called the "father of the fertilizer industry." He found that nitrogen is a key plant nutrient. He also created the Law of the Minimum. This described how individual nutrients affect crops.
Vladimir Markovnikov
Vladimir Markovnikov (born 1838) was a Russian scientist. He did most of his work at Kazan University in Russia. There, he studied under Alexander Butlerov. This lab was known as "the cradle of Russian organic chemistry." Markovnikov also studied chemistry in Germany for two years. His work in organic chemistry included developing Markovnikov's rule. This rule says that when hydrogen halides are added to alkenes and alkynes, the hydrogen will bond to the carbon with the most hydrogen atoms already. Products that follow this rule are called Markovnikov products. Those that don't are anti-Markovnikov products. Markovnikov's rule was an early example of how reactions choose specific places to happen. It is still important in the chemical industry today. Catalysts have been made to create anti-Markovnikov products. A key part of Markovnikov's rule is that it explains how things react based on how atoms are arranged. Many chemists at the time did not think chemical formulas showed the physical arrangement of atoms.
Mid-1800s Chemistry
In 1840, Germain Hess proposed Hess's law. This was an early idea about the law of conservation of energy. It says that energy changes in a chemical process depend only on the starting and ending materials. It does not matter how the change happens. In 1847, Hermann Kolbe made acetic acid from only inorganic sources. This further disproved vitalism. In 1848, William Thomson, 1st Baron Kelvin (Lord Kelvin) came up with absolute zero. This is the temperature where all molecular motion stops. In 1849, Louis Pasteur found that a certain form of tartaric acid was a mix of two mirror-image forms. This helped explain optical rotation and advanced stereochemistry. In 1852, August Beer proposed Beer's law. This explains how much light a mixture will absorb based on its makeup. This led to the analytical method called spectrophotometry. In 1855, Benjamin Silliman, Jr. started methods for petroleum cracking. This made the modern petrochemical industry possible.
Avogadro's idea became popular among chemists after his countryman Stanislao Cannizzaro showed its value in 1858. This was two years after Avogadro died. Cannizzaro's interests were originally in natural products and reactions of aromatic compounds. In 1853, he found that when benzaldehyde is treated with a strong base, both benzoic acid and benzyl alcohol are made. This is now called the Cannizzaro reaction. In his 1858 paper, Cannizzaro showed that using Avogadro's ideas could build a strong theory. This theory fit almost all the known evidence. For example, he showed that not all elementary gases have two atoms per molecule. Some were monatomic, most were diatomic, and a few were even more complex.
Another point of disagreement was the formulas for compounds of alkali metals (like sodium) and alkaline earth metals (like calcium). Because they were chemically similar, most chemists wanted to give them the same formula type. Cannizzaro argued that putting these metals in different groups helped. It removed some problems when using their physical properties to figure out atomic weights. Unfortunately, Cannizzaro's paper was first published only in Italian. It did not have much impact right away. The real breakthrough came at an international chemical congress in Karlsruhe, Germany, in September 1860. Most leading European chemists were there. The congress was organized by Kekulé, Wurtz, and others who agreed with Cannizzaro. Cannizzaro spoke in French. His clear and logical arguments deeply impressed the scientists. His friend Angelo Pavesi gave out Cannizzaro's paper at the end of the meeting. Many chemists later wrote about how important reading this document was. For example, Lothar Meyer wrote that after reading Cannizzaro's paper, "The scales seemed to fall from my eyes." Cannizzaro played a key role in winning the fight for reform. The system he supported, and which most leading chemists soon adopted, is mostly the same as what we use today.
Perkin, Crookes, and Nobel
In 1856, 18-year-old Sir William Henry Perkin was challenged by his professor. He tried to make quinine, a drug for malaria, from coal tar. In one try, Perkin used potassium dichromate to change aniline. Impurities reacted with the aniline and made a black solid. This seemed like a failed experiment. But when Perkin cleaned the flask with alcohol, he saw purple parts. A new synthetic dye, called mauveine or Perkin's mauve, was created by accident. Perkin's discovery started the dye industry. This was one of the first successful chemical industries.
German chemist August Kekulé von Stradonitz's most important contribution was his idea of organic structure. He wrote about it in two articles in 1857 and 1858. He explained it in detail in his very popular book Lehrbuch der organischen Chemie ("Textbook of Organic Chemistry"). The first part came out in 1859. Kekulé said that carbon atoms, which can form four chemical bonds, could link together. They could form what he called a "carbon chain" or "carbon skeleton." Other atoms like hydrogen, oxygen, nitrogen, and chlorine could join these chains. He believed chemists could figure out the detailed structure for simpler organic compounds. Kekulé was not the only one with these ideas. Scottish chemist Archibald Scott Couper published a similar theory around the same time. Russian chemist Aleksandr Butlerov also helped make structure theory clearer. But Kekulé's ideas were mostly the ones that became widely accepted.
British chemist and physicist William Crookes is known for his studies of cathode rays. These were key to developing atomic physics. He studied electrical discharges through a thin gas. He saw a dark space around the cathode, now called the Crookes dark space. He showed that cathode rays travel in straight lines. They also make light and heat when they hit certain materials. Crookes invented the Crookes tube. This was an early experimental tube to study cathode rays. When Robert Bunsen and Gustav Kirchhoff introduced spectrum analysis (1859–1860), Crookes used this new method. He studied selenium compounds. Bunsen and Kirchhoff had used spectroscopy to find caesium and rubidium. In 1861, Crookes used this process to find thallium in some selenium deposits. He kept working on this new element. He isolated it, studied its properties, and in 1873, found its atomic weight. While studying thallium, Crookes discovered the idea behind the Crookes radiometer. This device turns light into spinning motion. This idea has been used in many sensitive measuring tools.
In 1862, Alexander Parkes showed off Parkesine. This was one of the first synthetic polymers. This discovery started the modern plastics industry. In 1864, Cato Maximilian Guldberg and Peter Waage built on Claude Louis Berthollet's ideas. They proposed the law of mass action. In 1865, Johann Josef Loschmidt figured out the number of molecules in a mole. This was later called Avogadro's number.
In 1865, August Kekulé, partly based on Loschmidt's work, found the structure of benzene. It was a six-carbon ring with alternating single and double bonds. Kekulé's new idea for benzene's ring structure was debated a lot. But no better theory replaced it. This theory helped the German chemical industry grow a lot in the late 1800s. Kekulé is also famous for making clear what aromatic compounds are. These are compounds based on the benzene molecule. In 1865, Adolf von Baeyer started working on indigo dye. This was a big step in modern industrial organic chemistry. It changed the dye industry.
Swedish chemist and inventor Alfred Nobel found that mixing nitroglycerin with an absorbent substance like kieselguhr (diatomaceous earth) made it safer. He patented this mixture in 1867 as dynamite. Later, Nobel mixed nitroglycerin with other compounds. He found a good recipe combining another explosive nitrate. This made a clear, jelly-like substance. It was a more powerful explosive than dynamite. This substance, Gelignite, was patented in 1876. Many similar mixtures followed.
Mendeleev's Periodic Table
A big step in understanding the known chemical elements was Dmitri Mendeleev's creation of the first modern periodic table. Mendeleev, a Russian chemist, felt there was an order to the elements. He spent over 13 years gathering data and developing the idea. He wanted to help his students understand the field better. Mendeleev found that when all known chemical elements were put in order of increasing atomic weight, they showed a repeating pattern of properties. Mendeleev's law allowed him to build a systematic periodic table of the 66 elements known then. He published it in Principles of Chemistry in 1869. His first Periodic Table arranged elements by increasing atomic weight and grouped them by similar properties.
Mendeleev strongly believed in his periodic law. He suggested changes to the accepted atomic weights of some elements. In his 1871 periodic table, he predicted where unknown elements would go. He also predicted their properties. He even predicted the properties of three elements not yet found. He called them ekaboron (Eb), ekaaluminium (Ea), and ekasilicon (Es). These predictions were very accurate for scandium, gallium, and germanium. These elements later filled the spots Mendeleev assigned.
At first, chemists were not very interested in the periodic system. But when the predicted elements were discovered, especially gallium in 1875, scandium in 1879, and germanium in 1886, it gained wide acceptance. Many of his predictions were proven true during his lifetime. This made Mendeleev famous as the founder of the periodic law. His organization was better than earlier attempts. These included Alexandre-Émile Béguyer de Chancourtois's telluric helix (1862). Also, John Newlands proposed the law of octaves (1864). And Lothar Meyer developed an early periodic table with 28 elements (1864). Mendeleev's table did not include any of the noble gases. They had not been discovered yet. Slowly, the periodic law and table became the main framework for much of chemical theory. By the time Mendeleev died in 1907, he was known worldwide. He had received many honors and awards.
In 1873, Jacobus Henricus van 't Hoff and Joseph Achille Le Bel independently developed a model of chemical bonding. It explained Pasteur's chirality experiments. It also gave a physical reason for optical activity in chiral compounds. Van 't Hoff's publication, about 3-dimensional chemical structures, helped start stereochemistry. His idea of the "asymmetrical carbon atom" explained why many isomers existed. These could not be explained by older formulas. He also pointed out the link between optical activity and an asymmetrical carbon atom.
Josiah Willard Gibbs

American mathematical physicist J. Willard Gibbs's work on thermodynamics was key. It turned physical chemistry into a strict, logical science. From 1876 to 1878, Gibbs worked on thermodynamics principles. He applied them to complex chemical reactions. He discovered chemical potential. This is like the "fuel" that makes chemical reactions happen. In 1876, he published his most famous work, "On the Equilibrium of Heterogeneous Substances." This book gathered his work on thermodynamics and physical chemistry. It introduced the idea of free energy. This explained the physical basis of chemical balance. In these writings, Gibbs started his theories of matter phases. He saw each state of matter as a phase and each substance as a component. Gibbs took all the factors in a chemical reaction – temperature, pressure, energy, volume, and entropy. He put them into one simple equation called Gibbs' phase rule.
In this paper, he made perhaps his most important contribution. He introduced the idea of free energy, now called Gibbs free energy. Gibbs free energy shows how a system naturally tries to lower its energy and increase its disorder (or entropy). Gibbs's method lets a researcher calculate the change in free energy in a process, like a chemical reaction. It also shows how fast it will happen. Since almost all chemical and many physical processes involve such changes, his work greatly affected these sciences. In 1877, Ludwig Boltzmann used statistics to explain many physical and chemical ideas. These included entropy and how fast molecules move in gases. With Boltzmann and James Clerk Maxwell, Gibbs created a new part of physics called statistical mechanics. He even made up the term. It explains the laws of thermodynamics using the statistical properties of many particles. Gibbs also worked on applying Maxwell's equations to problems in physical optics. Gibbs's explanation of thermodynamics laws from the statistical properties of systems with many particles was in his important textbook, Elementary Principles in Statistical Mechanics, published in 1902. In that book, Gibbs reviewed the link between thermodynamics laws and the statistical theory of molecular motions. The way a Fourier series can go past the original function at certain points is called the Gibbs phenomenon.
Late 19th Century Advances
German engineer Carl von Linde invented a way to liquefy gases in large amounts. This formed the basis for modern refrigeration. It also helped scientific research at low temperatures and very high vacuums. He developed a refrigerator using dimethyl ether (1874) and one using ammonia (1876). Linde's refrigerators were the first designed with exact calculations for efficiency. In 1895, he built a large plant to make liquid air. Six years later, he found a way to separate pure liquid oxygen from liquid air. This led to industries widely using oxygen (for example, in making steel).
In 1883, Svante Arrhenius developed an ion theory. It explained how electrolytes conduct electricity. In 1884, Jacobus Henricus van 't Hoff published Études de Dynamique chimique. This was an important study on chemical kinetics. In this work, van 't Hoff entered the field of physical chemistry. Very important was his development of the general link between heat change and how equilibrium shifts with temperature. At a constant volume, the balance in a system will move to oppose the temperature change. So, lowering the temperature makes heat. Increasing it absorbs heat. This idea of mobile equilibrium was later (1885) put in a general form by Henry Louis Le Chatelier. He extended it to include changes in volume for pressure changes. The van 't Hoff-Le Chatelier principle, or simply Le Chatelier's principle, explains how dynamic chemical equilibria react to outside changes.
In 1884, Hermann Emil Fischer suggested the structure of purine. This is a key part of many biomolecules. He later made it in 1898. He also started working on the chemistry of glucose and related sugars. In 1885, Eugen Goldstein named the cathode ray, which was later found to be electrons. He also named the canal ray, which was later found to be positive hydrogen ions (protons). The year 1885 also saw J. H. van 't Hoff's book on dilute solutions. Here he showed that the "osmotic pressure" in very dilute solutions is related to the concentration and temperature. This pressure could be shown by a formula similar to the gas pressure formula, but with a coefficient 'i'. He also found the value of 'i' using different methods. This included vapor pressure and François-Marie Raoult's results on freezing point lowering. So, van 't Hoff proved that thermodynamic laws work not only for gases but also for dilute solutions. His pressure laws, made general by Arrhenius's theory of electrolytic dissociation, are very important in natural sciences. In 1893, Alfred Werner found the octahedral structure of cobalt complexes. This started the field of coordination chemistry.
Ramsay's Discovery of Noble Gases
The most famous discoveries of Scottish chemist William Ramsay were in inorganic chemistry. Ramsay was curious about British physicist John Strutt, 3rd Baron Rayleigh's 1892 discovery. Rayleigh found that the atomic weight of nitrogen in chemical compounds was lower than nitrogen in the air. Rayleigh thought this was due to a light gas in nitrogen compounds. Ramsay suspected a heavy, unknown gas in atmospheric nitrogen. Using two ways to remove all known gases from air, Ramsay and Lord Rayleigh announced in 1894 that they had found a monatomic, chemically inactive gas. It made up almost 1 percent of the atmosphere. They named it argon.
The next year, Ramsay released another inactive gas from a mineral called cleveite. This turned out to be helium, which was only known from the sun's spectrum before. In his 1896 book, The Gases of the Atmosphere, Ramsay showed that the positions of helium and argon in the periodic table suggested at least three more noble gases might exist. In 1898, Ramsay and British chemist Morris W. Travers isolated these elements—called neon, krypton, and xenon—from air. They turned them into a liquid state at low temperature and high pressure. Sir William Ramsay worked with Frederick Soddy. In 1903, they showed that alpha particles (helium nuclei) were constantly made during the radioactive decay of radium. Ramsay won the 1904 Nobel Prize for Chemistry. This was for his "services in the discovery of the inert gaseous elements in the air, and his determination of their place in the periodic system."
In 1897, J. J. Thomson discovered the electron using the cathode ray tube. In 1898, Wilhelm Wien showed that canal rays (streams of positive ions) can be bent by magnetic fields. The amount of bending depends on their mass-to-charge ratio. This discovery led to the analytical method called mass spectrometry in 1912.
Marie and Pierre Curie

Marie Skłodowska-Curie was a Polish-born French physicist and chemist. She is famous for her groundbreaking work on radioactivity. She and her husband are seen as starting the nuclear age with their research. Marie was very interested in the work of Henri Becquerel. He was a French physicist who found in 1896 that uranium gave off rays like X-rays. Marie Curie started studying uranium in late 1897. She thought that "the emission of rays by the compounds of uranium is a property of the metal itself—that it is an atomic property of the element uranium independent of its chemical or physical state." This was a revolutionary idea. It created the field of atomic physics. The Curies made up the word radioactivity to describe this.
Pierre and Marie explored radioactivity further. They worked to separate substances in uranium ores. They used an electrometer to measure radiation. This helped them find tiny amounts of unknown radioactive elements. Working with the mineral pitchblende, they found a new radioactive element in 1898. They named it polonium, after Marie's home country, Poland. On December 21, 1898, the Curies found another radioactive material in pitchblende. They told the French Academy of Sciences on December 26. They suggested the new element be called radium. The Curies then worked to isolate polonium and radium from natural compounds. They wanted to prove they were new elements. In 1902, the Curies announced they had made a small amount of pure radium. This showed it was a unique chemical element. It took them three years to isolate radium. But they were never able to isolate polonium. Besides finding two new elements and ways to isolate radioactive isotopes, Curie led the first studies on treating neoplasms (tumors) using radioactive isotopes. She, Henri Becquerel, and her husband, Pierre Curie, won the 1903 Nobel Prize for Physics. She was the only winner of the 1911 Nobel Prize for Chemistry. She was the first woman to win a Nobel Prize. She is the only woman to win the award in two different fields.
While working with Marie to get pure substances from ores, Pierre focused on the physical study of the new radiations. He used magnetic fields to study the rays from radium. He proved there were positively charged, negatively charged, and neutral particles. Ernest Rutherford later called these alpha, beta, and gamma rays. Pierre then studied these radiations with a calorimetry. He also saw the effects of radium on the body. This opened the way for radium therapy. Among Pierre Curie's discoveries was that magnetic substances lost their magnetic behavior above a certain temperature. This is called the "Curie point." He was elected to the Academy of Sciences in 1905. In 1903, he and Marie received the Royal Society's Davy Medal. He also won the Nobel Prize for Physics with Marie and Becquerel. He died in 1906 after being hit by a carriage in Paris. His complete works were published in 1908.
Ernest Rutherford

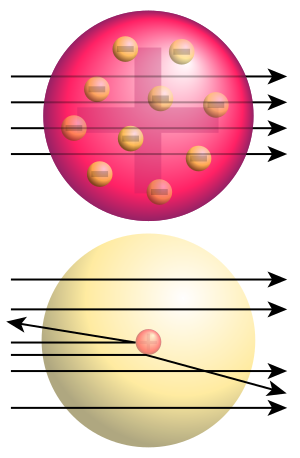
New Zealand-born chemist and physicist Ernest Rutherford is known as "the father of nuclear physics." Rutherford is best known for naming alpha, beta, and gamma to classify different kinds of radioactive "rays." These were not well understood at his time. (Alpha and beta rays are particle beams. Gamma rays are a form of high-energy electromagnetic radiation.) Rutherford bent alpha rays with electric and magnetic fields in 1903. Working with Frederick Soddy, Rutherford explained that radioactivity happens because elements change into other elements. This is now known to involve nuclear reactions.
He also saw that the strength of radioactivity of an element decreased over a regular time. He called the time it took to halve the "half-life." In 1901 and 1902, he worked with Frederick Soddy. They proved that atoms of one radioactive element would naturally turn into another. They did this by shooting out a piece of the atom at high speed. In 1906, at the University of Manchester, Rutherford oversaw an experiment. His students Hans Geiger (known for the Geiger counter) and Ernest Marsden did it. In the Geiger–Marsden experiment, a beam of alpha particles hit a very thin gold foil. Based on the old "plum pudding model" of the atom, the alpha particles should have all passed through. Or they should have only bent a little.
However, the actual results surprised Rutherford. Many alpha particles passed through as expected. But many others bent at small angles. Some even bounced back to the source. They saw that a very small number of particles bent at angles much larger than 90 degrees. The gold foil experiment showed big bends for a few particles. Rutherford realized that because some alpha particles bent or bounced back, the atom must have a small, dense center with a positive charge. Rutherford later called this positive center the "atomic nucleus." The alpha particles either hit this center directly or passed close enough to be affected by its positive charge. Since many other particles passed through the gold foil, the positive center had to be very small compared to the rest of the atom. This meant the atom is mostly empty space. From his results, Rutherford created a model of the atom like the solar system. This is known as the Rutherford model. Like planets, electrons orbited a central, sun-like nucleus. For his work with radiation and the atomic nucleus, Rutherford won the 1908 Nobel Prize in Chemistry.
20th Century Chemistry
In 1903, Mikhail Tsvet invented chromatography. This is an important way to analyze chemicals. In 1904, Hantaro Nagaoka suggested an early model of the atom. It had electrons orbiting a dense, heavy nucleus. In 1905, Fritz Haber and Carl Bosch developed the Haber process for making ammonia. This was a huge step in industrial chemistry. It had big effects on agriculture. The Haber process combines nitrogen and hydrogen to make ammonia in large amounts. This ammonia is used for fertilizer and explosives. Half the world's current food production depends on this method for fertilizer. Haber, with Max Born, suggested the Born–Haber cycle. This is a way to figure out the energy holding an ionic solid together. Haber has also been called the "father of chemical warfare" for his work on poisonous gases during World War I.

In 1905, Albert Einstein explained Brownian motion. This clearly proved atomic theory. Leo Baekeland invented bakelite, one of the first successful plastics. In 1909, American physicist Robert Andrews Millikan measured the charge of single electrons very accurately. He did this with the oil drop experiment. He measured the electric charges on tiny falling water (and later oil) droplets. His study showed that any droplet's charge was a multiple of a basic value—the electron's charge. This confirmed that all electrons have the same charge and mass. Starting in 1912, he spent years proving Albert Einstein's idea about energy and frequency. He provided the first direct support for Planck's constant in the photoelectric effect. In 1923, Millikan won the Nobel Prize for Physics.
In 1909, S. P. L. Sørensen created the pH concept. He also developed ways to measure acidity. In 1911, Antonius Van den Broek suggested that elements on the periodic table should be organized by their positive nuclear charge, not atomic weight. In 1911, the first Solvay Conference was held in Brussels. Many top scientists of the time attended. In 1912, William Henry Bragg and William Lawrence Bragg proposed Bragg's law. They started the field of X-ray crystallography. This is an important tool for figuring out the crystal structure of substances. In 1912, Peter Debye used the idea of a molecular dipole. This described how charge is unevenly spread in some molecules.
Otto Hahn
Otto Hahn was a German chemist. He was a pioneer in radioactivity and radiochemistry. He played a big part in discovering nuclear fission. He also made nuclear chemistry a scientific field. Hahn, Lise Meitner, and Fritz Strassmann found radioactive isotopes of radium, thorium, protactinium, and uranium. He also discovered atomic recoil and nuclear isomerism. He pioneered rubidium–strontium dating. In 1938, Hahn, Meitner, and Strassmann discovered nuclear fission. In their second paper on nuclear fission in February 1939, Hahn and Strassmann predicted that more neutrons would be released during fission. This opened the door to a nuclear chain reaction. Hahn received the 1944 Nobel Prize for Chemistry for these discoveries. Nuclear fission was the basis for nuclear reactors and nuclear weapons.
Niels Bohr
In 1913, Niels Bohr, a Danish physicist, brought ideas from quantum mechanics to atomic structure. He proposed what is now called the Bohr model of the atom. In this model, electrons only exist in specific circular orbits around the nucleus. These are like rungs on a ladder. The Bohr Model is like a tiny solar system. Negatively charged electrons orbit a small, positively charged nucleus. This is similar to planets orbiting the Sun.
In the Bohr model, electron orbits have a fixed size and energy. The energy levels are "quantized." This means only certain orbits with certain sizes are allowed. Orbits in between simply don't exist. The energy of an orbit is linked to its size. The lowest energy is in the smallest orbit. Bohr also said that light is absorbed or given off when an electron moves from one orbit to another. Because only certain electron orbits are allowed, light given off when an electron jumps from a high energy state to a low one creates a unique emission spectrum for each element. Bohr later won the Nobel Prize in physics for this work.
Niels Bohr also worked on the idea of complementarity. This says that an electron can be seen in two ways that seem to contradict each other, but both are true. Electrons can be seen as waves or particles. He thought that an incoming particle would hit the nucleus and create an excited compound nucleus. This was the basis of his liquid drop model. It later provided a theory for nuclear fission after it was discovered by chemists Otto Hahn and Fritz Strassman. Physicists Lise Meitner and Otto Frisch explained and named it.
In 1913, Henry Moseley, building on Van den Broek's idea, introduced the concept of atomic number. This helped fix some problems with Mendeleev's periodic table, which was based on atomic weight. The peak of Frederick Soddy's career was in 1913. He came up with the idea of isotopes. This said that some elements exist in two or more forms. They have different atomic weights but are chemically the same. He is remembered for proving isotopes exist for some radioactive elements. He is also credited with discovering the element protactinium in 1917. In 1913, J. J. Thomson expanded on Wien's work. He showed that charged subatomic particles can be separated by their mass-to-charge ratio. This technique is called mass spectrometry.
Gilbert N. Lewis and Chemical Bonds
American physical chemist Gilbert N. Lewis helped create the foundation of valence bond theory. He was important in developing a theory of bonding based on the number of electrons in the outermost "valence" shell of an atom. In 1902, Lewis tried to explain valence to his students. He drew atoms as cubes with electrons at each corner. This "cubic atom" explained the eight groups in the periodic table. It showed his idea that chemical bonds form when electrons are transferred. This gives each atom a full set of eight outer electrons (an "octet").
Lewis's theory of chemical bonding kept changing. In 1916, he published his important article "The Atom of the Molecule." It suggested that a chemical bond is a pair of electrons shared by two atoms. Lewis's model said that a classic chemical bond is when two bonded atoms share a pair of electrons. Lewis introduced "electron dot diagrams" in this paper. These showed the electron structures of atoms and molecules. Now called Lewis structures, they are in almost every basic chemistry book.
Soon after his 1916 paper, Lewis worked on military research. He did not return to chemical bonding until 1923. Then, he summarized his model in a short book called Valence and the Structure of Atoms and Molecules. His renewed interest was largely due to American chemist Irving Langmuir. Langmuir popularized and explained Lewis's model between 1919 and 1921. Langmuir later introduced the term covalent bond. In 1921, Otto Stern and Walther Gerlach established the idea of quantum mechanical spin in subatomic particles.
For cases where no sharing was involved, Lewis developed the electron pair theory of acids and base in 1923. Lewis redefined an acid as any atom or molecule with an incomplete octet. This meant it could accept electrons from another atom. Bases were electron donors. His theory is known as the concept of Lewis acids and bases. In 1923, G. N. Lewis and Merle Randall published Thermodynamics and the Free Energy of Chemical Substances. This was the first modern book on chemical thermodynamics.
In the 1920s, Lewis's model of the electron-pair bond was quickly adopted. It was used in organic and coordination chemistry. In organic chemistry, this was mainly due to British chemists Arthur Lapworth, Robert Robinson, Thomas Lowry, and Christopher Ingold. In coordination chemistry, Lewis's bonding model was promoted by American chemist Maurice Huggins and British chemist Nevil Sidgwick.
Quantum Mechanics and Chemistry
  |
|
  |
|
| From left to right, top row: Louis de Broglie (1892–1987) and Wolfgang Pauli (1900–58); second row: Erwin Schrödinger (1887–1961) and Werner Heisenberg (1901–76) |
Some people think quantum chemistry began with the discovery of the Schrödinger equation and its use for the hydrogen atom in 1926. However, the 1927 article by Walter Heitler and Fritz London is often seen as the first big step. This was the first time quantum mechanics was used for the diatomic hydrogen molecule. It explained the chemical bond. In the next few years, many scientists made progress. These included Edward Teller, Robert S. Mulliken, Max Born, J. Robert Oppenheimer, Linus Pauling, Erich Hückel, Douglas Hartree, and Vladimir Aleksandrovich Fock.
Still, some doubted if quantum mechanics could be used for complex chemical systems. Around 1930, Paul Dirac described the situation: "The basic physical laws for much of physics and all of chemistry are known. But applying these laws exactly leads to equations that are too complicated to solve. So, we need to develop approximate practical methods using quantum mechanics. These can explain the main features of complex atomic systems without too much calculation."
So, the quantum mechanical methods from the 1930s and 1940s are often called theoretical molecular or atomic physics. This shows they were more about applying quantum mechanics to chemistry and spectroscopy. They were less about answering specific chemical questions. In 1951, an important paper in quantum chemistry was Clemens C. J. Roothaan's paper on Roothaan equations. This opened the way to solving equations for small molecules like hydrogen or nitrogen. These calculations were done using tables of integrals. These were computed on the most advanced computers of the time.
In the 1940s, many physicists moved from molecular or atomic physics to nuclear physics. This included J. Robert Oppenheimer and Edward Teller. Glenn T. Seaborg was an American nuclear chemist. He was best known for finding and isolating transuranium elements (elements heavier than uranium). He shared the 1951 Nobel Prize for Chemistry with Edwin Mattison McMillan. This was for their separate discoveries of transuranium elements. Seaborgium was named after him. This made him one of the few people to have an element named after them while they were alive.
Molecular Biology and Biochemistry
By the mid-20th century, physics and chemistry were largely combined. Chemical properties were explained by the electronic structure of the atom. Linus Pauling's book The Nature of the Chemical Bond used quantum mechanics to figure out bond angles in complex molecules. However, even though some ideas from quantum mechanics could predict some chemical features for important biological molecules, they were mostly rules and observations. They were not strict, quantitative methods until the end of the 20th century.
This practical approach succeeded in 1953. James Watson and Francis Crick figured out the double helix structure of DNA. They built models using what they knew about the chemistry of DNA's parts. They also used X-ray diffraction patterns from Rosalind Franklin. This discovery led to a huge amount of research into the biochemistry of life.
In the same year, the Miller–Urey experiment showed that basic parts of proteins, simple amino acids, could be made from simpler molecules. This was done in a simulation of early Earth conditions. This first attempt by chemists to study possible processes in the lab helped start a lot of research into the origins of life.
In 1983, Kary Mullis invented a method to make many copies of DNA outside a living thing. This is called the polymerase chain reaction (PCR). It changed how DNA is handled in labs. PCR could make specific pieces of DNA. It made sequencing DNA of organisms possible. This led to the huge human genome project.
An important piece of the double helix puzzle was solved by one of Pauling's students, Matthew Meselson, and Frank Stahl. Their work (Meselson–Stahl experiment) is called "the most beautiful experiment in biology." They used a method that sorted molecules by weight. Nitrogen atoms are part of DNA. So, they were labeled and tracked during DNA copying in bacteria.
Late 20th Century Discoveries
In 1970, John Pople developed the Gaussian program. This made computational chemistry calculations much easier. In 1971, Yves Chauvin explained how olefin metathesis reactions work. In 1975, Karl Barry Sharpless and his team found reactions that control the specific arrangement of atoms in molecules. These included Sharpless epoxidation, Sharpless asymmetric dihydroxylation, and Sharpless oxyamination.
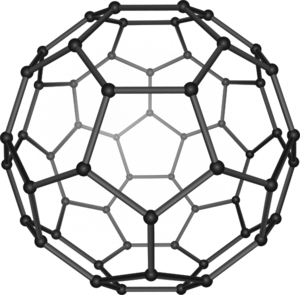
In 1985, Harold Kroto, Robert Curl, and Richard Smalley discovered fullerenes. These are large carbon molecules that look like the geodesic dome designed by architect R. Buckminster Fuller. In 1991, Sumio Iijima used electron microscopy to find a type of cylindrical fullerene called a carbon nanotube. Earlier work on this had been done as early as 1951. This material is important in nanotechnology. In 1994, K. C. Nicolaou and his group, and Robert A. Holton and his group, completed the first total synthesis of Taxol. In 1995, Eric Cornell and Carl Wieman made the first Bose–Einstein condensate. This substance shows quantum mechanical properties on a large scale.
Mathematics and Chemistry
Before the 20th century, chemistry was defined as the science of matter's nature and its changes. It was different from physics, which did not deal with such big changes in matter. Also, unlike physics, chemistry was mostly a descriptive and experimental science until the late 19th century. Even though chemists developed a strong quantitative base with atomic and molecular weights, they used less advanced math. Some even did not want to use math in chemistry. For example, the philosopher Auguste Comte wrote in 1830: "Any attempt to use mathematical methods in chemistry must be seen as deeply illogical and against the spirit of chemistry.... if mathematical analysis ever became important in chemistry – which is happily almost impossible – it would cause a quick and widespread decline of that science."
However, in the second half of the 19th century, things started to change. August Kekulé wrote in 1867: "I expect that someday we will find a mathematical and mechanical explanation for what we now call atoms. This will explain their properties."
What Chemistry Studies
As our understanding of matter has grown, so has chemistry's understanding of itself. This ongoing process includes what chemistry studies, its terms, goals, and reach. Also, the growth of scientific groups and networks that support chemical research are very important. They help create, share, and use chemical knowledge.
Chemical Industry
The late 19th century saw a huge increase in using petroleum from the earth. This was for making many chemicals. It largely replaced whale oil, coal tar, and other older materials. Large-scale production and refinement of petroleum provided raw materials. These were used for liquid fuels like gasoline and diesel. They also made solvents, lubricants, asphalt, waxes, and many modern materials. These include synthetic fibers, plastics, paints, detergents, pharmaceuticals, adhesives, and ammonia for fertilizer. Many of these needed new catalysts and chemical engineering to be made cheaply.
In the mid-20th century, controlling the electron structure of semiconductor materials became very precise. This was done by making large pieces of very pure single crystals of silicon and germanium. Carefully controlling their chemical makeup by adding other elements made the solid-state transistor possible in 1951. This also allowed for tiny integrated circuits for electronic devices, especially computers.
|
See also
 In Spanish: Historia de la química para niños
In Spanish: Historia de la química para niños
Histories and Timelines
- Atomic theory
- Cupellation
- History of chromatography
- History of electrochemistry
- History of the molecule
- History of molecular biology
- History of physics
- History of science and technology
- History of the periodic table
- History of thermodynamics
- History of energy
- History of molecular theory
- History of materials science
- List of years in science
- Nobel Prize in chemistry
- Timeline of atomic and subatomic physics
- Timeline of chemical elements discoveries
- Timeline of chemistry
- Timeline of materials technology
- Timeline of thermodynamics, statistical mechanics, and random processes
- The Chemical History of a Candle
- The Mystery of Matter: Search for the Elements (PBS film)
Famous Chemists
listed chronologically:
- List of chemists
- Robert Boyle, 1627–1691
- Joseph Black, 1728–1799
- Joseph Priestley, 1733–1804
- Carl Wilhelm Scheele, 1742–1786
- Antoine Lavoisier, 1743–1794
- Alessandro Volta, 1745–1827
- Jacques Charles, 1746–1823
- Claude Louis Berthollet, 1748–1822
- Amedeo Avogadro, 1776–1856
- Joseph-Louis Gay-Lussac, 1778–1850
- Humphry Davy, 1778–1829
- Jöns Jacob Berzelius, inventor of modern chemical notation, 1779–1848
- Justus von Liebig, 1803–1873
- Louis Pasteur, 1822–1895
- Stanislao Cannizzaro, 1826–1910
- Friedrich August Kekulé von Stradonitz, 1829–1896
- Dmitri Mendeleev, 1834–1907
- Josiah Willard Gibbs, 1839–1903
- J. H. van 't Hoff, 1852–1911
- William Ramsay, 1852–1916
- Svante Arrhenius, 1859–1927
- Walther Nernst, 1864–1941
- Marie Curie, 1867–1934
- Gilbert N. Lewis, 1875–1946
- Otto Hahn, 1879–1968
- Irving Langmuir, 1881–1957
- Linus Pauling, 1901–1994
- Glenn T. Seaborg, 1912–1999
- Robert Burns Woodward, 1917–1979
- Frederick Sanger, 1918–2013
- Geoffrey Wilkinson, 1921–1996
- Rudolph A. Marcus, 1923–
- George Andrew Olah, 1926–2017
- Elias James Corey, 1928–
- Akira Suzuki, 1930–
- Richard F. Heck, 1931–2015
- Harold Kroto, 1939–2016
- Jean-Marie Lehn, 1939–
- Peter Atkins, 1940–
- Barry Sharpless, 1941–
- Richard Smalley, 1943–2005
- Jean-Pierre Sauvage, 1944–